Localization of the genetic determinants of meiosis suppression in Daphnia pulex
- PMID: 18689898
- PMCID: PMC2535684
- DOI: 10.1534/genetics.107.084657
Localization of the genetic determinants of meiosis suppression in Daphnia pulex
Abstract
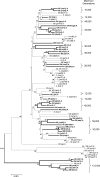
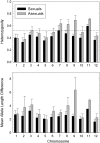
Although approximately 1 in 10,000 animal species is capable of parthenogenetic reproduction, the evolutionary causes and consequences of such transitions remain uncertain. The microcrustacean Daphnia pulex provides a potentially powerful tool for investigating these issues because lineages that are obligately asexual in terms of female function can nevertheless transmit meiosis-suppressing genes to sexual populations via haploid sperm produced by environmentally induced males. The application of association mapping to a wide geographic collection of D. pulex clones suggests that sex-limited meiosis suppression in D. pulex has spread westward from a northeastern glacial refugium, conveyed by a dominant epistatic interaction among the products of at least four unlinked loci, with one entire chromosome being inherited through males in a nearly nonrecombining fashion. With the enormous set of genomic tools now available for D. pulex, these results set the stage for the determination of the functional underpinnings of the conversion of meiosis to a mitotic-like mode of inheritance.
Figures




Similar articles
-
The role of hybridization in the origin and spread of asexuality in Daphnia.Mol Ecol. 2013 Sep;22(17):4549-61. doi: 10.1111/mec.12407. Epub 2013 Jul 23. Mol Ecol. 2013. PMID: 23879327 Free PMC article.
-
Population-genomic insights into the evolutionary origin and fate of obligately asexual Daphnia pulex.Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15740-5. doi: 10.1073/pnas.1313388110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959868 Free PMC article.
-
Hybridization and the Origin of Contagious Asexuality in Daphnia pulex.Mol Biol Evol. 2015 Dec;32(12):3215-25. doi: 10.1093/molbev/msv190. Epub 2015 Sep 8. Mol Biol Evol. 2015. PMID: 26351296 Free PMC article.
-
Methods for meiotic chromosome preparation, immunofluorescence, and fluorescence in situ hybridization in Daphnia pulex.Methods Mol Biol. 2009;558:235-49. doi: 10.1007/978-1-60761-103-5_14. Methods Mol Biol. 2009. PMID: 19685328 Review.
-
Using a meiosis detection toolkit to investigate ancient asexual "scandals" and the evolution of sex.Bioessays. 2008 Jun;30(6):579-89. doi: 10.1002/bies.20764. Bioessays. 2008. PMID: 18478537 Review.
Cited by
-
Laboratory generation of new parthenogenetic lineages supports contagious parthenogenesis in Artemia.PeerJ. 2014 Jun 17;2:e439. doi: 10.7717/peerj.439. eCollection 2014. PeerJ. 2014. PMID: 25024909 Free PMC article.
-
A population of sexual Daphnia pulex resists invasion by asexual clones.Proc Biol Sci. 2014 Aug 7;281(1788):20140564. doi: 10.1098/rspb.2014.0564. Proc Biol Sci. 2014. PMID: 24943366 Free PMC article.
-
Chromosome Unipolar Division and Low Expression of Tws May Cause Parthenogenesis of Rice Water Weevil (Lissorhoptrus oryzophilus Kuschel).Insects. 2021 Mar 24;12(4):278. doi: 10.3390/insects12040278. Insects. 2021. PMID: 33805047 Free PMC article.
-
Asexual Daphnia genomes expose something old, new, borrowed, and blue.Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15518-9. doi: 10.1073/pnas.1314088110. Epub 2013 Sep 23. Proc Natl Acad Sci U S A. 2013. PMID: 24062444 Free PMC article. No abstract available.
-
High mutational rates of large-scale duplication and deletion in Daphnia pulex.Genome Res. 2016 Jan;26(1):60-9. doi: 10.1101/gr.191338.115. Epub 2015 Oct 30. Genome Res. 2016. PMID: 26518480 Free PMC article.
References
-
- Anger, M., P. Stein and R. M. Schultz, 2005. CDC6 requirement for spindle formation during maturation of mouse oocytes. Biol. Reprod. 72 188–194. - PubMed
-
- Barton, N. H., and B. Charlesworth, 1998. Why sex and recombination? Science 281 1986–1990. - PubMed
-
- Bopp, D., C. Schutt, J. Puro, H. Huang and R. Nothiger, 1999. Recombination and disjunction in female germ cells of Drosophila depend on the germline activity of the gene sex-lethal. Development 126 5785–5794. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

