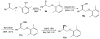A practical and efficient route for the highly enantioselective synthesis of mexiletine analogues and novel beta-thiophenoxy and pyridyl ethers
- PMID: 18690744
- PMCID: PMC2668572
- DOI: 10.1021/jo801181d
A practical and efficient route for the highly enantioselective synthesis of mexiletine analogues and novel beta-thiophenoxy and pyridyl ethers
Abstract
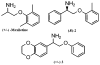
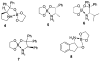
A practical and efficient procedure for the enantioselective synthesis of mexiletine analogues with use of 10% of spiroborate ester 6 as chirality transfer agent is presented. A variety of mexiletine analogues were prepared in good yield with excellent enantioselectivities (91-97% ee) from readily available starting materials. The developed methodology was also successfully applied for the synthesis of novel beta-amino ethers containing thiophenyl and pyridyl fragments.
Figures
References
-
- De Luca A, Natuzzi F, Desaphy J-F, Loni G, Lentini G, Franchini C, Tortorella V, Conte Camerino D. Mol Pharmacol. 2000;57:268–277. - PubMed
- De Luca A, Natuzzi F, Duranti A, Lentini G, Franchini C, Tortorella V, Conte Camerino D. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:777–787. - PubMed
- De Luca A, Pierno S, Natuzzi F, Franchini C, Duranti A, Lentini G, Tortorella V, Jockush H, Conte Camerino D. J Pharmacol Exp Ther. 1997;282:93–100. - PubMed
- Fenster PE, Comes KA. Pharmacotherapy. 1986;6:1–9. - PubMed
-
- Turgeon J, Uprichard ACG, Belanger PM, Harron DWC, Grech-Belanger OJ. Pharm Pharmacol. 1991;43:630–635. - PubMed
-
- Hill RJ, Duff HJ, Sheldon RS. Mol Pharmacol. 1988;34:659–663. - PubMed
-
- DeLuca A, Natuzzi F, Lentini G, Franchini C, Tortorella V, Conte Camerino D. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;352:653–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources