Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes
- PMID: 18691124
- PMCID: PMC2904242
- DOI: 10.2174/138920308785132668
Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes
Abstract
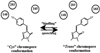
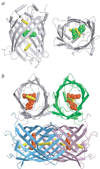
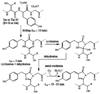
Green fluorescent protein (GFP) from jellyfish Aequorea victoria is the most extensively studied and widely used in cell biology protein. GFP-like proteins constitute a fast growing family as several naturally occurring GFP-like proteins have been discovered and enhanced mutants of Aequorea GFP have been created. These mutants differ from wild-type GFP by conformational stability, quantum yield, spectroscopic properties (positions of absorption and fluorescence spectra) and by photochemical properties. GFP-like proteins are very diverse, as they can be not only green, but also blue, orange-red, far-red, cyan, and yellow. They also can have dual-color fluorescence (e.g., green and red) or be non-fluorescent. Some of them possess kindling property, some are photoactivatable, and some are photoswitchable. This review is an attempt to characterize the main color groups of GFP-like proteins, describe their structure and mechanisms of chromophore formation, systemize data on their conformational stability and summarize the main trends of their utilization as markers and biosensors in cell and molecular biology.
Figures




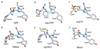




References
-
- Lukyanov KA, Fradkov AF, Gurskaya NG, Matz MV, Labas YA, Savitsky AP, Markelov ML, Zaraisky AG, Zhao X, Fang Y, Tan W, Lukyanov SA. J Biol Chem. 2000;275:25879–25882. - PubMed
-
- Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Trends Biochem Sci. 1995;20:448–455. - PubMed
-
- Zacharias DA, Baird GS, Tsien RY. Curr Opin Neurobiol. 2000;10:416–421. - PubMed
-
- Ward WW, Cormier MJ. J Biol Chem. 1979;254:781–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
