P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis
- PMID: 18691411
- PMCID: PMC2518548
- DOI: 10.1186/1742-2094-5-33
P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis
Abstract
Background: The purinergic receptor P2x7 is expressed on myeloid cells as well as on CNS glial cells, and P2x7 activation has been shown to increase both glial and T-cell activation. These properties suggest a role in the development of autoimmune disease including multiple sclerosis.
Methods: The animal model of MS, experimental autoimmune encephalomyelitis (EAE) using myelin oligodendrocyte glycoprotein (MOG) peptide residues 35-55 was induced in wildtype C57BL6 mice and in P2x7 deficient mice ('P2x7 mice') that were backcrossed to C57BL6 mice. Disease progression was monitored by appearance of clinical signs, immunocytochemical staining to assess brain inflammation and neuronal damage, and by measurement of Tcell cytokine production.
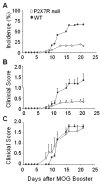
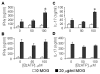
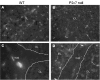
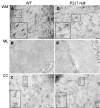
Results: The incidence of EAE disease in P2x7 mice was reduced 4-fold compared to the wildtype mice; however the P2x7 mice that became ill had similar days of onset and clinical scores as the wildtype mice. Splenic T-cells isolated from P2x7 null mice produced greater IFNgamma and IL-17 (from 3 to 12 fold greater levels) than wildtype cells, however cytokine production from P2x7 derived cells was not increased by a selective P2x7 agonist as was cytokine production from wildtype cells. Although infiltrating cells were detected in brains of both the P2x7 and wildtype mice, astroglial activation and axonal damage was reduced versus wildtype mice, and the distribution of astroglial activation was markedly distinct in the two strains. In contrast, microglial activation was similar in the two strains.
Conclusion: P2x7 deficiency resulted in compensatory changes leading to increased T-cell cytokine production, and activated T-cells were detected in the brains of P2x7 null mice with no clinical signs. However, the greatly reduced incidence of disease suggests that an initiating event is absent in these mice, and points to a role for astroglial P2x7 in development of EAE disease.
Figures



References
-
- Marcoli M, Cervetto C, Paluzzi P, Guarnieri S, Alloisio S, Thellung S, Nobile M, Maura G. P2X(7) pre-synaptic receptors in adult rat cerebrocortical nerve terminals: a role in ATP-induced glutamate release. J Neurochem. 2008 - PubMed
-
- Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. - DOI - PMC - PubMed
-
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev. 2005;48:157–165. doi: 10.1016/j.brainresrev.2004.12.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

