Chimeric alphavirus vaccine candidates for chikungunya
- PMID: 18692107
- PMCID: PMC2571998
- DOI: 10.1016/j.vaccine.2008.07.054
Chimeric alphavirus vaccine candidates for chikungunya
Abstract
Chikungunya virus (CHIKV) is an emerging alphavirus that has caused major epidemics in India and islands off the east coast of Africa since 2005. Importations into Europe and the Americas, including one that led to epidemic transmission in Italy during 2007, underscore the risk of endemic establishment elsewhere. Because there is no licensed human vaccine, and an attenuated Investigational New Drug product developed by the U.S. Army causes mild arthritis in some vaccinees, we developed chimeric alphavirus vaccine candidates using either Venezuelan equine encephalitis attenuated vaccine strain TC-83, a naturally attenuated strain of eastern equine encephalitis virus (EEEV), or Sindbis virus as a backbone and the structural protein genes of CHIKV. All vaccine candidates replicated efficiently in cell cultures, and were highly attenuated in mice. All of the chimeras also produced robust neutralizing antibody responses, although the TC-83 and EEEV backbones appeared to offer greater immunogenicity. Vaccinated mice were fully protected against disease and viremia after CHIKV challenge.
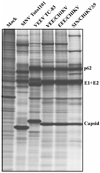
Figures






References
-
- Karabatsos N. International Catalog of Arboviruses Including Certain Other Viruses of Vertebrates. 4th ed. San Antonio: American Society of Tropical Medicine and Hygiene; 1985. - PubMed
-
- Carey DE. Chikungunya and dengue: a case of mistaken identity? J Hist Med Allied Sci. 1971;26(3):243–262. - PubMed
-
- Halstead SB, Udomsakdi S, Scanlon JE, Rohitayodhin S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. V. Epidemiologic observations outside Bangkok. American Journal of Tropical Medicine & Hygiene. 1969;18(6):1022–1033. - PubMed
-
- Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. American Journal of Tropical Medicine & Hygiene. 1969;18(6):997–1021. - PubMed
-
- Rao TR. Recent epidemics caused by chikungunya virus in India, 1963–1965. Scientific Culture. 1966;32:215.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

