Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds
- PMID: 18692775
- PMCID: PMC2605408
- DOI: 10.1016/j.chom.2008.05.018
Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds
Abstract
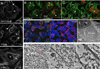
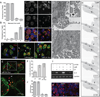
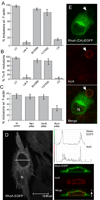
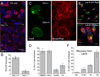
The obligate intracellular bacterial pathogen Chlamydia trachomatis replicates within a large vacuole or "inclusion" that expands as bacteria multiply but is maintained as an intact organelle. Here, we report that the inclusion is encased in a scaffold of host cytoskeletal structures made up of a network of F-actin and intermediate filaments (IF) that act cooperatively to stabilize the pathogen-containing vacuole. Formation of F-actin at the inclusion was dependent on RhoA, and its disruption led to the disassembly of IFs, loss of inclusion integrity, and leakage of inclusion contents into the host cytoplasm. In addition, IF proteins were processed by the secreted chlamydial protease CPAF to form filamentous structures at the inclusion surface with altered structural properties. We propose that Chlamydia has co-opted the function of F-actin and IFs to stabilize the inclusion with a dynamic, structural scaffold while minimizing the exposure of inclusion contents to cytoplasmic innate immune-surveillance pathways.
Figures
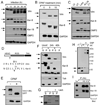
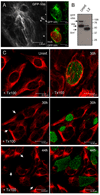
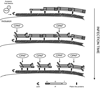
Comment in
-
Chlamydia weave a protective cloak spun of actin and intermediate filaments.Cell Host Microbe. 2008 Aug 14;4(2):93-5. doi: 10.1016/j.chom.2008.07.006. Cell Host Microbe. 2008. PMID: 18692768
Similar articles
-
Cross Talk between ARF1 and RhoA Coordinates the Formation of Cytoskeletal Scaffolds during Chlamydia Infection.mBio. 2021 Dec 21;12(6):e0239721. doi: 10.1128/mBio.02397-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903051 Free PMC article.
-
Chlamydia weave a protective cloak spun of actin and intermediate filaments.Cell Host Microbe. 2008 Aug 14;4(2):93-5. doi: 10.1016/j.chom.2008.07.006. Cell Host Microbe. 2008. PMID: 18692768
-
A meta-analysis of affinity purification-mass spectrometry experimental systems used to identify eukaryotic and chlamydial proteins at the Chlamydia trachomatis inclusion membrane.J Proteomics. 2020 Feb 10;212:103595. doi: 10.1016/j.jprot.2019.103595. Epub 2019 Nov 21. J Proteomics. 2020. PMID: 31760040 Free PMC article.
-
Got mutants? How advances in chlamydial genetics have furthered the study of effector proteins.Pathog Dis. 2021 Feb 4;79(2):ftaa078. doi: 10.1093/femspd/ftaa078. Pathog Dis. 2021. PMID: 33512479 Free PMC article. Review.
-
Chlamydia trachomatis and its interaction with the cellular retromer.Int J Med Microbiol. 2018 Jan;308(1):197-205. doi: 10.1016/j.ijmm.2017.10.006. Epub 2017 Oct 26. Int J Med Microbiol. 2018. PMID: 29122514 Review.
Cited by
-
Inclusion Membrane Growth and Composition Are Altered by Overexpression of Specific Inclusion Membrane Proteins in Chlamydia trachomatis L2.Infect Immun. 2021 Jun 16;89(7):e0009421. doi: 10.1128/IAI.00094-21. Epub 2021 Jun 16. Infect Immun. 2021. PMID: 33875478 Free PMC article.
-
Sulforaphane promotes chlamydial infection by suppressing mitochondrial protein oxidation and activation of complement C3.Protein Sci. 2019 Jan;28(1):216-227. doi: 10.1002/pro.3536. Protein Sci. 2019. PMID: 30367535 Free PMC article.
-
The Chlamydia trachomatis IncM Protein Interferes with Host Cell Cytokinesis, Centrosome Positioning, and Golgi Distribution and Contributes to the Stability of the Pathogen-Containing Vacuole.Infect Immun. 2023 Apr 18;91(4):e0040522. doi: 10.1128/iai.00405-22. Epub 2023 Mar 6. Infect Immun. 2023. PMID: 36877064 Free PMC article.
-
Chlamydial protease-like activity factor--insights into immunity and vaccine development.J Reprod Immunol. 2009 Dec;83(1-2):179-84. doi: 10.1016/j.jri.2009.05.007. Epub 2009 Oct 23. J Reprod Immunol. 2009. PMID: 19853923 Free PMC article. Review.
-
Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections.Int J Mol Sci. 2020 Jun 30;21(13):4675. doi: 10.3390/ijms21134675. Int J Mol Sci. 2020. PMID: 32630064 Free PMC article. Review.
References
-
- Aktories K, Just I. Clostridial Rho-inhibiting protein toxins. Curr Top Microbiol Immunol. 2005;291:113–145. - PubMed
-
- Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 2003;5:793–802. - PubMed
-
- Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006;119:350–359. - PubMed
-
- Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol. 2004;2:530–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

