Platelet factor 4 mediates inflammation in experimental cerebral malaria
- PMID: 18692777
- PMCID: PMC2603442
- DOI: 10.1016/j.chom.2008.07.003
Platelet factor 4 mediates inflammation in experimental cerebral malaria
Abstract
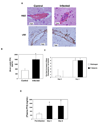
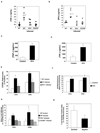
Cerebral malaria (CM) is a major complication of Plasmodium falciparum infection in children. The pathogenesis of CM involves vascular inflammation, immune stimulation, and obstruction of cerebral capillaries. Platelets have a prominent role in both immune responses and vascular obstruction. We now demonstrate that the platelet-derived chemokine, platelet factor 4 (PF4)/CXCL4, promotes the development of experimental cerebral malaria (ECM). Plasmodium-infected red blood cells (RBCs) activated platelets independently of vascular effects, resulting in increased plasma PF4. PF4 or chemokine receptor CXCR3 null mice had less severe ECM, including decreased T cell recruitment to the brain, and platelet depletion or aspirin treatment reduced the development of ECM. We conclude that Plasmodium-infected RBCs can directly activate platelets, and platelet-derived PF4 then contributes to immune activation and T cell trafficking as part of the pathogenesis of ECM.
Figures


Similar articles
-
Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria.PLoS One. 2010 May 3;5(5):e10413. doi: 10.1371/journal.pone.0010413. PLoS One. 2010. PMID: 20454664 Free PMC article.
-
Beta interferon suppresses the development of experimental cerebral malaria.Infect Immun. 2011 Apr;79(4):1750-8. doi: 10.1128/IAI.00810-10. Epub 2011 Jan 18. Infect Immun. 2011. PMID: 21245265 Free PMC article.
-
Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum.Science. 2012 Dec 7;338(6112):1348-51. doi: 10.1126/science.1228892. Science. 2012. PMID: 23224555
-
Cerebral malaria pathogenesis: revisiting parasite and host contributions.Future Microbiol. 2012 Feb;7(2):291-302. doi: 10.2217/fmb.11.155. Future Microbiol. 2012. PMID: 22324996 Review.
-
Cerebral malaria pathogenesis: Dissecting the role of CD4+ and CD8+ T-cells as major effectors in disease pathology.Int Rev Immunol. 2024;43(5):309-325. doi: 10.1080/08830185.2024.2336539. Epub 2024 Apr 15. Int Rev Immunol. 2024. PMID: 38618863 Review.
Cited by
-
Platelet-Specific Chemokines Contribute to the Pathogenesis of Acute Lung Injury.Am J Respir Cell Mol Biol. 2017 Feb;56(2):261-270. doi: 10.1165/rcmb.2015-0245OC. Am J Respir Cell Mol Biol. 2017. PMID: 27755915 Free PMC article.
-
Genetic variants in platelet factor 4 modulate inflammatory and platelet activation biomarkers.Circ Cardiovasc Genet. 2012 Aug 1;5(4):412-21. doi: 10.1161/CIRCGENETICS.111.961813. Epub 2012 Jul 4. Circ Cardiovasc Genet. 2012. PMID: 22763266 Free PMC article.
-
Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases?Mediators Inflamm. 2015;2015:435783. doi: 10.1155/2015/435783. Epub 2015 Feb 26. Mediators Inflamm. 2015. PMID: 25814789 Free PMC article. Review.
-
Extracellular Sphingomyelinase Rv0888 of Mycobacterium tuberculosis Contributes to Pathological Lung Injury of Mycobacterium smegmatis in Mice via Inducing Formation of Neutrophil Extracellular Traps.Front Immunol. 2018 Apr 4;9:677. doi: 10.3389/fimmu.2018.00677. eCollection 2018. Front Immunol. 2018. PMID: 29670633 Free PMC article.
-
Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review.Medicina (Kaunas). 2023 Apr 11;59(4):746. doi: 10.3390/medicina59040746. Medicina (Kaunas). 2023. PMID: 37109704 Free PMC article.
References
-
- Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. - PubMed
-
- Chakravorty SJ, Craig A. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol. 2005;84:15–27. - PubMed
-
- Eslin DE, Zhang C, Samuels KJ, Rauova L, Zhai L, Niewiarowski S, Cines DB, Poncz M, Kowalska MA. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–3180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

