Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays
- PMID: 18693260
- PMCID: PMC2814162
- DOI: 10.1002/bies.20804
Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays
Abstract
Microscale techniques have been applied to biological assays for nearly two decades, but haven't been widely integrated as common tools in biological laboratories. The significant differences between several physical phenomena at the microscale versus the macroscale have been exploited to provide a variety of new types of assays (such as gradient production or spatial cell patterning). However, the use of these devices by biologists seems to be limited by issues regarding biological validation, ease of use, and the limited available readouts for assays done using microtechnology. Critical validation work has been done recently that highlights the current challenges for microfluidic methods and suggest ways in which future devices might be improved to better integrate with biological assays. With more validation and improved designs, microscale techniques hold immense promise as a platform to study aspects of cell biology that are not possible using current macroscale techniques.
Figures
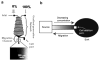
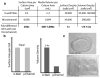
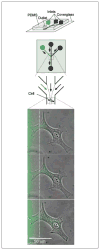

Comment in
-
Comment on "Microfluidics meets cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays".Bioessays. 2009 Feb;31(2):255. doi: 10.1002/bies.200900016. Bioessays. 2009. PMID: 19204980 No abstract available.
References
-
- Sims CE, Allbritton NL. Analysis of single mammalian cells on-chip. Lab On A Chip. 2007;7:423–440. - PubMed
-
- Meyvantsson I, Warrick JW, Hayes S, Skoien A, Beebe DJ. Automated cell culture in high density tubeless microfluidic device arrays. Lab Chip. 2008;8(5):717–24. - PubMed
-
- Tan W, Desai TA. Microfluidic patterning of cells in extracellular matrix biopolymers: effects of \ channel size, cell type, and matrix composition on pattern integrity. Tissue Eng. 2003;9:255–567. - PubMed
-
- Fisher RJ, Peattie RA. Controlling tissue microenvironments: biomimetics, transport phenomena, and reacting systems. Adv Biochem Eng Biotechnol. 2007;103:1–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

