Differential sorting of human parathyroid hormone after transduction of mouse and rat salivary glands
- PMID: 18694295
- PMCID: PMC2705888
- DOI: 10.1089/hum.2008.079
Differential sorting of human parathyroid hormone after transduction of mouse and rat salivary glands
Abstract
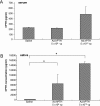
Gene transfer to salivary glands leads to abundant secretion of transgenic protein into either saliva or the bloodstream. This indicates significant clinical potential, depending on the route of sorting. The aim of this study was to probe the sorting characteristics of human parathyroid hormone (hPTH) in two animal models for salivary gland gene transfer. PTH is a key hormone regulating calcium levels in the blood. A recombinant serotype 5 adenoviral vector carrying the hPTH cDNA was administered to the submandibular glands of mice and rats. Two days after delivery, high levels of hPTH were found in the serum of mice, leading to elevated serum calcium levels. Only low amounts of hPTH were found in the saliva. Two days after vector infusion into rats, a massive secretion of hPTH was measured in saliva, with little secretion into serum. Confocal microscopy showed hPTH in the glands, localized basolaterally in mice and apically in rats. Submandibular gland transduction was effective and the produced hPTH was biologically active in vivo. Whereas hPTH sorted toward the basolateral side in mice, in rats hPTH was secreted mainly at the apical side. These results indicate that the interaction between hPTH and the cell sorting machinery is different between mouse and rat salivary glands. Detailed studies in these two species should result in a better understanding of cellular control of transgenic secretory protein sorting in this tissue.
Figures



Similar articles
-
Production and sorting of transgenic, modified human parathyroid hormone in vivo in rat salivary glands.Biochem Biophys Res Commun. 2010 Jan 1;391(1):768-72. doi: 10.1016/j.bbrc.2009.11.135. Epub 2009 Nov 26. Biochem Biophys Res Commun. 2010. PMID: 19944067 Free PMC article.
-
Human parathyroid hormone is secreted primarily into the bloodstream after rat parotid gland gene transfer.Hum Gene Ther. 2011 Jan;22(1):84-92. doi: 10.1089/hum.2010.097. Epub 2011 Jan 3. Hum Gene Ther. 2011. PMID: 20977345 Free PMC article.
-
In vivo secretion of the mouse immunoglobulin G Fc fragment from rat submandibular glands.J Gene Med. 2009 Jul;11(7):580-7. doi: 10.1002/jgm.1340. J Gene Med. 2009. PMID: 19424985 Free PMC article.
-
Parathyroid hormone metabolites in renal failure: bioactivity and clinical implications.Semin Dial. 2002 May-Jun;15(3):196-201. doi: 10.1046/j.1525-139x.2002.00053.x. Semin Dial. 2002. PMID: 12100458 Review.
-
Salivary epithelial cells: an unassuming target site for gene therapeutics.Int J Biochem Cell Biol. 2010 Jun;42(6):773-7. doi: 10.1016/j.biocel.2010.02.012. Epub 2010 Feb 26. Int J Biochem Cell Biol. 2010. PMID: 20219693 Free PMC article. Review.
Cited by
-
In vivo veritas: the power of in situ manipulation of cells in a living animal. Focus on "Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals".Am J Physiol Cell Physiol. 2009 Dec;297(6):C1333-5. doi: 10.1152/ajpcell.00437.2009. Epub 2009 Oct 7. Am J Physiol Cell Physiol. 2009. PMID: 19812369 Free PMC article. No abstract available.
-
Production and sorting of transgenic, modified human parathyroid hormone in vivo in rat salivary glands.Biochem Biophys Res Commun. 2010 Jan 1;391(1):768-72. doi: 10.1016/j.bbrc.2009.11.135. Epub 2009 Nov 26. Biochem Biophys Res Commun. 2010. PMID: 19944067 Free PMC article.
-
Transgenic α-1-antitrypsin secreted into the bloodstream from salivary glands is biologically active.Oral Dis. 2011 Jul;17(5):476-83. doi: 10.1111/j.1601-0825.2010.01775.x. Epub 2010 Dec 2. Oral Dis. 2011. PMID: 21122036 Free PMC article.
-
Expression and secretion of human proinsulin-B10 from mouse salivary glands: implications for the treatment of type I diabetes mellitus.PLoS One. 2013;8(3):e59222. doi: 10.1371/journal.pone.0059222. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554999 Free PMC article.
-
Human parathyroid hormone is secreted primarily into the bloodstream after rat parotid gland gene transfer.Hum Gene Ther. 2011 Jan;22(1):84-92. doi: 10.1089/hum.2010.097. Epub 2011 Jan 3. Hum Gene Ther. 2011. PMID: 20977345 Free PMC article.
References
-
- Alimohammadi M. Bjorklund P. Hallgren A. Pontynen N. Szinnai G. Shikama N. Keller M.P. Ekwall O. Kinkel S.A. Husebye E.S. Gustafsson J. Rorsman F. Peltonen L. Betterle C. Perheentupa J. Akerstrom G. Westin G. Scott H.S. Hollander G.A. Kampe O. Autoimmune polyendocrine syndrome type 1 and NALP5, parathyroid autoantigen. N. Engl. J. Med. 2008;358:1018–1028. - PubMed
-
- Arvan P. Halban P.A. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. - PubMed
-
- Baum B.J. Berkman M.E. Marmary Y. Goldsmith C.M. Baccaglini L. Wang S.L. Wellner R.B. Hoque A.T.M.S. Atkinson J.C. Yamagishi H. Kagami H. Parlow A.F. Chao J.L. Polarized secretion of transgene products from salivary glands in vivo. Hum. Gene Ther. 1999;10:2789–2797. - PubMed
-
- Baum B.J. Voutetakis A. Wang J.H. Salivary glands: Novel target sites for gene therapeutics. Trends Mol. Med. 2004;10:585–590. - PubMed
-
- Blair H.C. Zaidi M. Osteoclastic differentiation and function regulated by old and new pathways. Rev. Endocr. Metab. Disord. 2006;7:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

