Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis
- PMID: 18694335
- PMCID: PMC2729545
- DOI: 10.1086/591461
Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis
Abstract
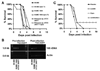
Environmental isolates of the fungus Rhizopus have been shown to harbor a bacterial endosymbiont (Burkholderia) that produces rhixozin, a plant mycotoxin. We sought to define the role of rhizoxin production by endosymbionts in the pathogenesis of mucormycosis. Endosymbiotic bacteria were identified by polymerase chain reaction in 15 (54%) of 28 clinical isolates of Zygomycetes, with 33% of the bacterial strains showing 87% identity to Burkholderia 16S rDNA. The presence of rhizoxin in myclial extracts from fungi harboring bacteria was confirmed by high-performance liquid chromatography analysis. However, fungal strains with or without endosymbionts did not differ in their ability to cause endothelial cell injury in vitro, nor did antibiotic-mediated eradication of endosymbionts and rhizoxin production decrease the virulence of fungal strains in mice or flies. In summary, although bacterial endosymbiosis is widely detected in clinical isolates of Zygomycetes, including Rhizopus oryzae strains, we found no evidence that bacterial endosymbionts and rhizoxin contribute to the pathogenesis of mucormycosis in the models studied.
Conflict of interest statement
Potential conflicts of interest: none reported.
Figures




References
-
- Kwon-Chung KJ, Bennett JE. Medical mycology. Philadelphia: Lea & Febiger; 1992. Mucormycosis; pp. 524–559.
-
- Sugar AM. Agent of mucormycosis and related species. In: Mandell G, Bennett J, Dolin R, editors. Principles and practices of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 2311–2321.
-
- Ibrahim AS, Edwards JEJ, Filler SG. Zygomycosis. In: Dismukes WE, Pappas PG, Sobel JD, editors. Clinical mycology. New York: Oxford University Press; 2003. pp. 41–251.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

