Gene expression time-series analysis of camptothecin effects in U87-MG and DBTRG-05 glioblastoma cell lines
- PMID: 18694480
- PMCID: PMC2556695
- DOI: 10.1186/1476-4598-7-66
Gene expression time-series analysis of camptothecin effects in U87-MG and DBTRG-05 glioblastoma cell lines
Abstract
Background: The clinical efficacy of camptothecin (CPT), a drug specifically targeting topoisomerase I (TopoI), is under evaluation for the treatment of malignant gliomas. Due to the high unresponsiveness of these tumours to chemotherapy, it would be very important to study the signalling network that drives camptothecin outcome in this type of cancer cells. To address this issue, we had previously compared the expression profile of human U87-MG glioblastoma cells with that of a CPT-resistant counterpart, giving evidence that the development of a robust inflammatory response was the main transcriptional effect associated with CPT resistance. Here we report time-related changes and cell line specific patterns of gene expression after CPT treatment by using two p53 wild-type glioblastoma cell lines, U87-MG and DBTRG-05, with different sensitivities to TopoI inhibition.
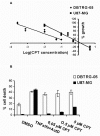
Results: First, we demonstrated that CPT treatment brings the two cell lines to completely different outcomes: accelerated senescence in U87-MG and apoptosis in DBTRG-05 cells. Then, to understand the different susceptibility to CPT, we used oligo-microarray to identify the genes whose expression was regulated during a time-course treatment, ranging from 2 h to 72 h. The statistical analysis of microarray data by MAANOVA (MicroArray ANalysis Of VAriance) showed much less modulated genes in apoptotic DBTRG-05 cells (155) with respect to the senescent U87-MG cells (3168), where the number of down-regulated genes largely exceeded that of the up-regulated ones (80% vs. 20%). Despite this great difference, the two data-sets showed a large overlapping (60% circa) mainly due to the expression of early stress responsive genes. The use of High-Throughput GoMINER and EASE tools, for functional analysis of significantly enriched GO terms, highlighted common cellular processes and showed that U87-MG and DBTRG-05 cells shared many GO terms, which are related to the down-regulation of cell cycle and mitosis and to the up-regulation of cell growth inhibition and DNA damage.Furthermore, the down-regulation of MYC and DP1 genes, which act as key transcription factors in cell growth control, together with the inhibition of BUB1, BUB3 and MAD2 mRNAs, which are known to be involved in the spindle checkpoint pathway, were specifically associated with the execution of senescence in U87-MG cells and addressed as critical factors that could drive the choice between different CPT-inducible effectors programs. In U87-MG cells we also found inflammation response and IL1-beta induction, as late transcriptional effects of Topo I treatment but these changes were only partially involved in the senescence development, as shown by IL1-beta gene silencing.
Conclusion: By comparing the transcription profile of two glioblastoma cell lines treated with camptothecin, we were able to identify the common cellular pathways activated upon Topo I inhibition. Moreover, our results helped in identifying some key genes whose expression seemed to be associated with the execution of senescence or apoptosis in U87-MG and DBTRG-05 cells, respectively.
Figures
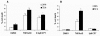

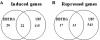


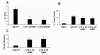
References
-
- Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. - PubMed
-
- Liu LF, Desai SD, LI T, Mao Y, Sun M, Sim S. Mechanism of action of camptothecin. Ann N Y Acad Sci. 2000;922:1–26. - PubMed
-
- Carson JP, Zhang N, Frampton GM, Gerry NP, Lenburg ME, Christman MF. Pharmacogenomic identification of targets for adjuvant therapy with the topoisomerase poison camptothecin. Cancer Res. 2004;64:2096–2104. - PubMed
-
- Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, Hendrickson EA, Balan KV, Pantazis P, Wyche JH. Role of p21 in Apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277:17154–17160. - PubMed
-
- Wang Y, Zhu S, Cloughesy TF, Liau LM, Mischel PS. p53 disruption profoundly alters the response of human glioblastoma cells to DNA topoisomerase I inhibition. Oncogene. 2004;23:1283–1290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

