CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease
- PMID: 18694559
- PMCID: PMC3987787
- DOI: 10.1016/j.devcel.2008.07.004
CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease
Abstract
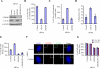
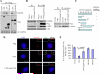
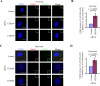
Primary cilia are nonmotile organelles implicated in signaling and sensory functions. Understanding how primary cilia assemble could shed light on the many human diseases caused by mutations in ciliary proteins. The centrosomal protein CP110 is known to suppress ciliogenesis through an unknown mechanism. Here, we report that CP110 interacts with CEP290--a protein whose deficiency is implicated in human ciliary disease--in a discrete complex separable from other CP110 complexes involved in regulating the centrosome cycle. Ablation of CEP290 prevents ciliogenesis without affecting centrosome function or cell-cycle progression. Interaction with CEP290 is absolutely required for the ability of CP110 to suppress primary cilia formation. Furthermore, CEP290 and CP110 interact with Rab8a, a small GTPase required for cilia assembly. Depletion of CEP290 interferes with localization of Rab8a to centrosomes and cilia. Our results suggest that CEP290 cooperates with Rab8a to promote ciliogenesis and that this function is antagonized by CP110.
Figures




References
-
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. - PubMed
-
- Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat. Rev. Genet. 2005;6:194–205. - PubMed
-
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 2007;8:451–463. - PubMed
-
- Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 2006;15:1847–1857. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

