Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning
- PMID: 18694564
- PMCID: PMC2581521
- DOI: 10.1016/j.devcel.2008.06.013
Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning
Abstract
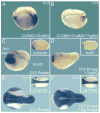
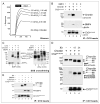
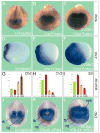
Vertebrate Crossveinless-2 (CV2) is a secreted protein that can potentiate or antagonize BMP signaling. Through embryological and biochemical experiments we find that: (1) CV2 functions as a BMP4 feedback inhibitor in ventral regions of the Xenopus embryo; (2) CV2 complexes with Twisted gastrulation and BMP4; (3) CV2 is not a substrate for tolloid proteinases; (4) CV2 binds to purified Chordin protein with high affinity (K(D) in the 1 nM range); (5) CV2 binds even more strongly to Chordin proteolytic fragments resulting from Tolloid digestion or to full-length Chordin/BMP complexes; (6) CV2 depletion causes the Xenopus embryo to become hypersensitive to the anti-BMP effects of Chordin overexpression or tolloid inhibition. We propose that the CV2/Chordin interaction may help coordinate BMP diffusion to the ventral side of the embryo, ensuring that BMPs liberated from Chordin inhibition by tolloid proteolysis cause peak signaling levels.
Figures


Comment in
-
Intriguing extracellular regulation of BMP signaling.Dev Cell. 2008 Aug;15(2):176-7. doi: 10.1016/j.devcel.2008.07.012. Dev Cell. 2008. PMID: 18694555
References
-
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008 in press. - PubMed
-
- Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, Rupp F. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem Biophys Res Commun. 2004;315:272–280. - PubMed
-
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. - PubMed
-
- Blitz IL, Chow KW, Chang C. Twisted gastrulation loss-of-function analyses support its role as a BMP inhibitor during early Xenopus embryogenesis. Development. 2003;130:4975–4988. - PubMed
-
- Blitz IL, Shimmi O, Wünnenberg-Stapleton K, O’Connor MB, Cho KW. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev Biol. 2000;223:120–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

