Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype
- PMID: 18694967
- PMCID: PMC2573340
- DOI: 10.1128/IAI.00395-08
Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype
Abstract
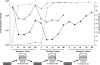
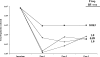
Bacteria adapt to environmental changes through high-frequency switches in expression of specific phenotypes. Localized hypermutation mediated by simple sequence repeats is an important mechanism of such phase variation (PV) in Neisseria meningitidis. Loss or gain of nucleotides in a poly(C) tract located in the reading frame results in switches in expression of lgtG and determines whether a glucose or a phosphoethanolamine (PEtn) is added at a specific position in the inner core lipopolysaccharide (LPS). Monoclonal antibody (MAb) B5 is bactericidal for N. meningitidis strain 8047 when PEtn is present in the inner core LPS and lgtG is switched "off." Escape from the bactericidal activity of this antibody was examined by subjecting strain 8047 to multiple cycles of growth in the presence of MAb B5 and human serum. Escape variants with alterations in the lgtG repeat tract rapidly accumulated in bacterial populations during selection with this antibody. Strain 8047 was outcompeted in this assay by the 8047 Delta mutS strain due to the elevated PV rate of this mismatch repair mutant and hence the greater proportion of preexisting phase variants of lgtG in the inoculum. This mutS mutant was also more virulent than strain 8047 during escape from passive protection by MAb B5 in an in vivo infant rat model of bacteremia. These results provide an example of how PV rates can modulate the occurrence and severity of infection and have important implications for understanding the evolution of bacterial fitness in species subject to environmental variations that occur during persistence within and transmission between hosts.
Figures






References
-
- Achtman, M., R. A. Wall, M. Bopp, B. Kusecek, G. Morelli, E. Saken, and M. Hassan-King. 1991. Variation in class 5 protein expression by serogroup A meningococci during a meningitis epidemic. J. Infect. Dis. 164375-382. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2003. Current protocols in molecular biology. John Wiley and Sons, Inc., Hoboken, NJ.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

