Identification of a hypervariable region containing new Legionella pneumophila Icm/Dot translocated substrates by using the conserved icmQ regulatory signature
- PMID: 18694969
- PMCID: PMC2546816
- DOI: 10.1128/IAI.00337-08
Identification of a hypervariable region containing new Legionella pneumophila Icm/Dot translocated substrates by using the conserved icmQ regulatory signature
Abstract
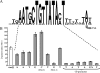
Legionella pneumophila is an intracellular pathogen that has been shown to utilize the Icm/Dot type IV secretion system for pathogenesis. This system was shown to be composed of Icm/Dot complex components, accessory proteins, and a large number of translocated substrates. In this study, comparison of the icmQ regulatory regions from many Legionella species revealed a conserved regulatory sequence that includes the icmQ -10 promoter element. Mutagenesis of this conserved regulatory element indicated that each of the nucleotides in it affects the level of expression of the icmQ gene but not in a uniform fashion. A genomic analysis discovered that four additional genes in L. pneumophila contain this conserved regulatory sequence, which was found to function similarly in these genes as well. Examination of these four genes indicated that they are dispensable for intracellular growth, but two of them were found to encode new Icm/Dot translocated substrates (IDTS). Comparison of the genomic regions encoding these two IDTS among the four available L. pneumophila genomic sequences indicated that one of these genes is located in a hypervariable genomic region, which was shown before to contain an IDTS-encoding gene. Translocation analysis that was performed for nine proteins encoded from this hypervariable genomic region indicated that six of them are new IDTS which are translocated into host cells in an Icm/Dot-dependent manner. Furthermore, a bioinformatic analysis indicated that additional L. pneumophila genomic regions that contain several neighboring IDTS-encoding genes are hypervariable in gene content.
Figures







References
-
- Bruggemann, H., C. Cazalet, and C. Buchrieser. 2006. Adaptation of Legionella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins. Curr. Opin. Microbiol. 986-94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

