TRPP2 and TRPV4 form a polymodal sensory channel complex
- PMID: 18695040
- PMCID: PMC2500130
- DOI: 10.1083/jcb.200805124
TRPP2 and TRPV4 form a polymodal sensory channel complex
Abstract
The primary cilium has evolved as a multifunctional cellular compartment that decorates most vertebrate cells. Cilia sense mechanical stimuli in various organs, but the molecular mechanisms that convert the deflection of cilia into intracellular calcium transients have remained elusive. Polycystin-2 (TRPP2), an ion channel mutated in polycystic kidney disease, is required for cilia-mediated calcium transients but lacks mechanosensitive properties. We find here that TRPP2 utilizes TRPV4 to form a mechano- and thermosensitive molecular sensor in the cilium. Depletion of TRPV4 in renal epithelial cells abolishes flow-induced calcium transients, demonstrating that TRPV4, like TRPP2, is an essential component of the ciliary mechanosensor. Because TRPV4-deficient zebrafish and mice lack renal cysts, our findings challenge the concept that defective ciliary flow sensing constitutes the fundamental mechanism of cystogenesis.
Figures
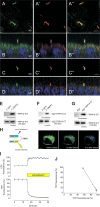


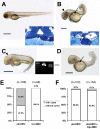

References
-
- Cuajungco, M.P., C. Grimm, K. Oshima, D. D'Hoedt, B. Nilius, A.R. Mensenkamp, R.J. Bindels, M. Plomann, and S. Heller. 2006. PACSINs bind to the TRPV4 cation channel. PACSIN 3 modulates the subcellular localization of TRPV4. J. Biol. Chem. 281:18753–18762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

