Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc
- PMID: 18695044
- PMCID: PMC2500138
- DOI: 10.1083/jcb.200711151
Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc
Abstract
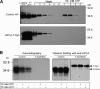
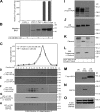
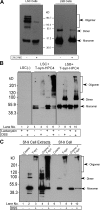
Regulatory pathways for protein glycosylation are poorly understood, but expression of branchpoint enzymes is critical. A key branchpoint enzyme is the T-synthase, which directs synthesis of the common core 1 O-glycan structure (T-antigen), the precursor structure for most mucin-type O-glycans in a wide variety of glycoproteins. Formation of active T-synthase, which resides in the Golgi apparatus, requires a unique molecular chaperone, Cosmc, encoded on Xq24. Cosmc is the only molecular chaperone known to be lost through somatic acquired mutations in cells. We show that Cosmc is an endoplasmic reticulum (ER)-localized adenosine triphosphate binding chaperone that binds directly to human T-synthase. Cosmc prevents the aggregation and ubiquitin-mediated degradation of the T-synthase. These results demonstrate that Cosmc is a molecular chaperone in the ER required for this branchpoint glycosyltransferase function and show that expression of the disease-related Tn antigen can result from deregulation or loss of Cosmc function.
Figures





References
-
- Ahner, A., and J.L. Brodsky. 2004. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 14:474–478. - PubMed
-
- Allen, A.C., P.S. Topham, S.J. Harper, and J. Feehally. 1997. Leucocyte beta 1,3 galactosyltransferase activity in IgA nephropathy. Nephrol. Dial. Transplant. 12:701–706. - PubMed
-
- Berger, E.G. 1999. Tn-syndrome. Biochim. Biophys. Acta. 1455:255–268. - PubMed
-
- Bergeron, J.J., M.B. Brenner, D.Y. Thomas, and D.B. Williams. 1994. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 19:124–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

