Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study
- PMID: 18695059
- PMCID: PMC3409562
- DOI: 10.1001/archneur.65.8.1091
Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study
Abstract
Background: The Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) score is commonly used, although the utility regarding this score in staging dementia severity is not well established.
Objective: To investigate the effectiveness of CDR-SOB scores in staging dementia severity compared with the global CDR score.
Design: Retrospective study.
Setting: Texas Alzheimer's Research Consortium minimum data set cohort.
Participants: A total of 1577 participants (110 controls, 202 patients with mild cognitive impairment, and 1265 patients with probable Alzheimer disease) were available for analysis.
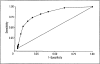
Main outcome measures: Receiver operating characteristic curves were generated from a derivation sample to determine optimal cutoff scores and ranges, which were then applied to the validation sample.
Results: Optimal ranges of CDR-SOB scores corresponding to the global CDR scores were 0.5 to 4.0 for a global score of 0.5, 4.5 to 9.0 for a global score of 1.0, 9.5 to 15.5 for a global score of 2.0, and 16.0 to 18.0 for a global score of 3.0. When applied to the validation sample, kappa scores ranged from 0.86 to 0.94 (P < .001 for all), with 93.0% of the participants falling within the new staging categories.
Conclusions: The CDR-SOB score compares well with the global CDR score for dementia staging. Owing to the increased range of values, the CDR-SOB score offers several advantages over the global score, including increased utility in tracking changes within and between stages of dementia severity. Interpretive guidelines for CDR-SOB scores are provided.
Figures
References
-
- Horowitz F. Developmental theory, prediction, and the developmental equation in follow-up research. In: Friedman S, Haywood HC, editors. Developmental Follow-up: Concepts, Domains, and Methods. Academic Press; San Diego. CA: 1994. pp. 27–44.
-
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: Mini-Mental State Examination and Clinical Dementia Rating. Am J Geriar Psychiatry. 2006;14(2):139–144. - PubMed
-
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154–1166. - PubMed
-
- Leber P. Guidelines for the Clinical Evaluahon of Antidementia Drugs. US Food and Drug Administration; Rockville. MD: 1990.
-
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

