Phosphorylation events and the modulation of aquaporin 2 cell surface expression
- PMID: 18695390
- PMCID: PMC3774073
- DOI: 10.1097/MNH.0b013e3283094eb1
Phosphorylation events and the modulation of aquaporin 2 cell surface expression
Abstract
Purpose of review: This review highlights the role of phosphorylation in the trafficking and targeting of aquaporin 2. Current knowledge will be put into the context of modulating the cell surface expression of aquaporin 2 by vasopressin in renal epithelial cells, which is critical for regulation of urinary concentration and control of fluid and electrolyte homeostasis.
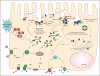
Recent findings: In addition to previously identified phosphorylation sites on aquaporin 2, new data have revealed three other serine residues in the C-terminus whose phosphorylation is altered by vasopressin. Several steps in aquaporin 2 recycling, including exocytosis and endocytosis, are coordinated by phosphorylation and dephosphorylation to regulate cell surface accumulation. Aquaporin 2 phosphorylation on serine 256 regulates aquaporin 2 association with proteins that are involved in trafficking, including hsc/hsp70 and myelin and lymphocyte-associated protein.
Summary: Aquaporin 2 trafficking is regulated by phosphorylation of serine 256 and other amino acid residues in its cytoplasmic domain. These events increase or decrease interaction of aquaporin 2 with key regulatory proteins to determine the cellular distribution and fate of aquaporin 2, both after vasopressin addition and under baseline conditions. Better understanding of these mechanisms may provide new therapeutic avenues for patients with X-linked nephrogenic diabetes insipidus, as well as providing basic cell biological information relevant to membrane trafficking processes in general.
Figures
References
-
- van Balkom BW, Savelkoul PJ, Markovich D, et al. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem. 2002;277:41473–41479. - PubMed
-
- Valenti G, Procino G, Carmosino M, et al. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci. 2000;113:1985–1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

