Inactivation of nitric oxide by uric acid
- PMID: 18696365
- PMCID: PMC2701227
- DOI: 10.1080/15257770802257952
Inactivation of nitric oxide by uric acid
Abstract
The 1980 identification of nitric oxide (NO) as an endothelial cell-derived relaxing factor resulted in an unprecedented biomedical research of NO and established NO as one of the most important cardiovascular, nervous and immune system regulatory molecule. A reduction in endothelial cell NO levels leading to "endothelial dysfunction" has been identified as a key pathogenic event preceding the development of hypertension, metabolic syndrome, and cardiovascular disease. The reduction in endothelial NO in cardiovascular disease has been attributed to the action of oxidants that either directly react with NO or uncouple its substrate enzyme. In this report, we demonstrate that uric acid (UA), the most abundant antioxidant in plasma, reacts directly with NO in a rapid irreversible reaction resulting in the formation of 6-aminouracil and depletion of NO. We further show that this reaction occurs preferentially with NO even in the presence of oxidants peroxynitrite and hydrogen peroxide and that the reaction is at least partially blocked by glutathione. This study shows a potential mechanism by which UA may deplete NO and cause endothelial dysfunction, particularly under conditions of oxidative stress in which UA is elevated and intracellular glutathione is depleted.
Figures


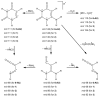


References
-
- Wu X, Muzny DM, Lee CC, Caskey CT. Two idependent mutational events in the loss of urate oxides during hominoid evolution. J Mol Evol. 1992;34:78–84. - PubMed
-
- Kuzkaya N, Weissmann N, Harrison DF, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. - PubMed
-
- Robinson KM, Morre JT, Beckman JS. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch Bioch Biophys. 2004;423:213–217. - PubMed
-
- Kand'ar P, Zakova P, Muzakova V. Monitoring of antioxidant antioxidant properties properties of uric uric acid acid in humans humans for a consideration consideration measuring measuring of levels levels of allantoin allantoin in plasma plasma by liquid liquid chromatographychromatography. Clin Chim Acta. 2006;365:249–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
