Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats
- PMID: 18697194
- PMCID: PMC2562302
- DOI: 10.1002/cne.21801
Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats
Abstract
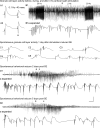
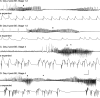
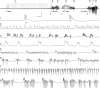
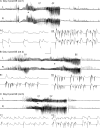
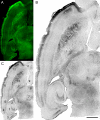
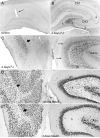
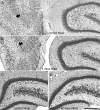
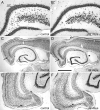
Hippocampal epileptogenesis is hypothesized to involve secondary mechanisms triggered by initial brain injury. Chemoconvulsant-induced status epilepticus has been used to identify secondary epileptogenic mechanisms under the assumption that a seizure-free, preepileptic "latent period" exists that is long enough to accommodate delayed mechanisms. The latent period is difficult to assess experimentally because early spontaneous seizures may be caused or influenced by residual chemoconvulsant that masks the true duration of the epileptogenic process. To avoid the use of chemoconvulsants and determine the latency to hippocampal epileptogenesis and clinical epilepsy, we developed an electrical stimulation-based method to evoke hippocampal discharges in awake rats and produce hippocampal injury and hippocampal-onset epilepsy reliably. Continuous video monitoring and granule cell layer recording determined whether hippocampal epileptogenesis develops immediately or long after injury. Bilateral perforant pathway stimulation for 3 hours evoked granule cell epileptiform discharges and convulsive status epilepticus with minimal lethality. Spontaneous stage 3-5 behavioral seizures reliably developed within 3 days poststimulation, and all 72 spontaneous behavioral seizures recorded in 10 animals were preceded by spontaneous granule cell epileptiform discharges. Histological analysis confirmed a reproducible pattern of limited hippocampal and extrahippocampal injury, including an extensive bilateral loss of hilar neurons throughout the hippocampal longitudinal axis. These results indicate that hippocampal epileptogenesis after convulsive status epilepticus is an immediate network defect coincident with neuron loss or other early changes. We hypothesize that the latent period is directly related and inversely proportional to the extent of neuron loss in brain regions involved in seizure initiation, spread, and clinical expression.
Figures
Similar articles
-
Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single "cryptic" episode of focal hippocampal excitation in awake rats.J Comp Neurol. 2010 Aug 15;518(16):3381-407. doi: 10.1002/cne.22406. J Comp Neurol. 2010. PMID: 20575073 Free PMC article.
-
No latency to dentate granule cell epileptogenesis in experimental temporal lobe epilepsy with hippocampal sclerosis.Epilepsia. 2018 Nov;59(11):2019-2034. doi: 10.1111/epi.14580. Epub 2018 Oct 19. Epilepsia. 2018. PMID: 30338519
-
Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse.J Comp Neurol. 2009 Jul 10;515(2):181-96. doi: 10.1002/cne.22059. J Comp Neurol. 2009. PMID: 19412934 Free PMC article.
-
Defining "epileptogenesis" and identifying "antiepileptogenic targets" in animal models of acquired temporal lobe epilepsy is not as simple as it might seem.Neuropharmacology. 2013 Jun;69:3-15. doi: 10.1016/j.neuropharm.2012.01.022. Epub 2012 Feb 4. Neuropharmacology. 2013. PMID: 22342985 Free PMC article. Review.
-
Abnormal dentate gyrus network circuitry in temporal lobe epilepsy.In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. PMID: 22787603 Free Books & Documents. Review.
Cited by
-
Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma.FASEB J. 2010 Mar;24(3):853-61. doi: 10.1096/fj.09-145870. Epub 2009 Nov 4. FASEB J. 2010. PMID: 19890018 Free PMC article.
-
Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies.Neurotherapeutics. 2014 Apr;11(2):373-84. doi: 10.1007/s13311-013-0250-1. Neurotherapeutics. 2014. PMID: 24425186 Free PMC article. Review.
-
Perirhinal cortex and temporal lobe epilepsy.Front Cell Neurosci. 2013 Aug 29;7:130. doi: 10.3389/fncel.2013.00130. Front Cell Neurosci. 2013. PMID: 24009554 Free PMC article. Review.
-
Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single "cryptic" episode of focal hippocampal excitation in awake rats.J Comp Neurol. 2010 Aug 15;518(16):3381-407. doi: 10.1002/cne.22406. J Comp Neurol. 2010. PMID: 20575073 Free PMC article.
-
Status Epilepticus Induced Spontaneous Dentate Gyrus Spikes: In Vivo Current Source Density Analysis.PLoS One. 2015 Jul 6;10(7):e0132630. doi: 10.1371/journal.pone.0132630. eCollection 2015. PLoS One. 2015. PMID: 26148195 Free PMC article.
References
-
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966;66:448–460. - PubMed
-
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. - PubMed
-
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. - PubMed
-
- Bertram EH, Cornett J. The ontogeny of seizures in a rat model of limbic epilepsy: evidence for a kindling process in the development of chronic spontaneous seizures. Brain Res. 1993;625:295–300. - PubMed
-
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

