Early participant attrition from clinical trials: role of trial design and logistics
- PMID: 18697847
- PMCID: PMC2723836
- DOI: 10.1177/1740774508094406
Early participant attrition from clinical trials: role of trial design and logistics
Abstract
Background: Participant attrition from randomized controlled trials reduces the statistical power of the study and can potentially introduce bias. Early identification of potential causes of attrition can help reduce patient attrition. We performed secondary analyses of two trials involving cancer patients.
Purpose: To identify predictors of attrition during two early phases, i.e., from consent to screening (Phase-1), and from screening to intake interview (Phase-2) in two clinical trials.
Methods: Cancer patients undergoing chemotherapy were asked to enroll in one of two clinical trials. In each trial the benefits of a cognitive behavioral intervention were compared with a psycho-educational intervention to assist patients to manage cancer and treatment-related symptoms. Following consent patients were screened for their symptoms' severity to determine their eligibility.
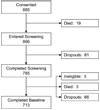
Results: Of the 885 consenters 785 completed screening and of the 782 eligible for participation, 713 completed intake interview. In the first phase, longer delays between consent and first contact attempt, lower levels of patient education, minority race, and prolonged duration of screening increased the likelihood of dropping out with a significantly stronger effect on minorities than white patients. In the second phase, low education, being a minority, longer screening delays, and impact of symptom severity on enjoyment of life significantly increased probability of attrition.
Limitations: Participant reported causes of attrition were not modeled; however, exclusion of patients who died during the time period of this research meant that most patients leaving the study made a conscious decision to do so.
Conclusions: To assure preservation of external validity, the time between consent and randomization into the arms of a trial must be held to a minimum. Delays between contacts and run in time, that may include screening patients to assure they will benefit from a trial, must be balanced against rates of attrition. Compressing intervals between contacts is particularly important to retain minorities.
Figures
References
-
- Jordhoy MS, Kaasa S, Fayers P, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med. 1999 June 1;13(4):299–310. - PubMed
-
- Young AF, Powers JR, Bell SL. Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health. 2006 Aug;30(4):353–361. - PubMed
-
- Twisk J, de Vente W. Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol. 2002 Apr;55(4):329–337. - PubMed
-
- Giuliano AR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, Ho RCS, et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000 Nov;10(8 Suppl):S22–S34. - PubMed
-
- Neumark DE, Stommel M, Given CW, Given BA. Research design and subject characteristics predicting nonparticipation in a panel survey of older families with cancer. Nurs Res. 2001 Nov–Dec;50(6):363–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources