Two highly related regulatory subunits of PP2A exert opposite effects on TGF-beta/Activin/Nodal signalling
- PMID: 18697906
- PMCID: PMC4940033
- DOI: 10.1242/dev.020842
Two highly related regulatory subunits of PP2A exert opposite effects on TGF-beta/Activin/Nodal signalling
Abstract
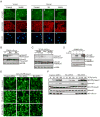
We identify Balpha (PPP2R2A) and Bdelta (PPP2R2D), two highly related members of the B family of regulatory subunits of the protein phosphatase PP2A, as important modulators of TGF-beta/Activin/Nodal signalling that affect the pathway in opposite ways. Knockdown of Balpha in Xenopus embryos or mammalian tissue culture cells suppresses TGF-beta/Activin/Nodal-dependent responses, whereas knockdown of Bdelta enhances these responses. Moreover, in Drosophila, overexpression of Smad2 rescues a severe wing phenotype caused by overexpression of the single Drosophila PP2A B subunit Twins. We show that, in vertebrates, Balpha enhances TGF-beta/Activin/Nodal signalling by stabilising the basal levels of type I receptor, whereas Bdelta negatively modulates these pathways by restricting receptor activity. Thus, these highly related members of the same subfamily of PP2A regulatory subunits differentially regulate TGF-beta/Activin/Nodal signalling to elicit opposing biological outcomes.
Figures


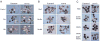



References
-
- Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280:42644–54. - PubMed
-
- Bajpai R, Makhijani K, Rao PR, Shashidhara LS. Drosophila Twins regulates Armadillo levels in response to Wg/Wnt signal. Development. 2004;131:1007–16. - PubMed
-
- Bardwell VJ, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–77. - PubMed
-
- Batut J, Howell M, Hill CS. Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-β ligands. Developmental Cell. 2007;12:261–274. - PubMed
-
- Bennett D, Alphey L. PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster. Nat Genet. 2002;31:419–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

