The effect of particle design on cellular internalization pathways
- PMID: 18697944
- PMCID: PMC2575324
- DOI: 10.1073/pnas.0801763105
The effect of particle design on cellular internalization pathways
Abstract
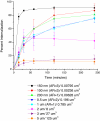
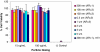
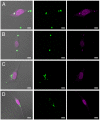
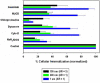
The interaction of particles with cells is known to be strongly influenced by particle size, but little is known about the interdependent role that size, shape, and surface chemistry have on cellular internalization and intracellular trafficking. We report on the internalization of specially designed, monodisperse hydrogel particles into HeLa cells as a function of size, shape, and surface charge. We employ a top-down particle fabrication technique called PRINT that is able to generate uniform populations of organic micro- and nanoparticles with complete control of size, shape, and surface chemistry. Evidence of particle internalization was obtained by using conventional biological techniques and transmission electron microscopy. These findings suggest that HeLa cells readily internalize nonspherical particles with dimensions as large as 3 mum by using several different mechanisms of endocytosis. Moreover, it was found that rod-like particles enjoy an appreciable advantage when it comes to internalization rates, reminiscent of the advantage that many rod-like bacteria have for internalization in nonphagocytic cells.
Conflict of interest statement
Conflict of interest statement: The research reported in this paper received partial financial support from a venture capital-backed company that Dr. Joseph M. DeSimone cofounded, Liquidia Technologies (
Figures


References
-
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Controlled Release. 2004;100:135–144. - PubMed
-
- Kabanov AV, Felgner PL, Seymour LW. Self-assembling Complexes for Gene Delivery. From Laboratory to Clinical Trial. Chichester: Wiley; 1998.
-
- Sarciaux JM, Acar L, Sado PA. Using microemulsion formulations for oral-drug delivery of therapeutic peptides. Int J Pharm. 1995;120:127–136.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

