Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa
- PMID: 18698342
- PMCID: PMC2488375
- DOI: 10.1371/journal.pone.0002921
Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa
Erratum in
- PLoS One. 2011;6(2). doi:10.1371/annotation/5284a0b6-62a9-484e-80a4-a08c59b3b17c
Abstract
Background: Vaccination with a recombinant modified vaccinia Ankara expressing antigen 85A from Mycobacterium tuberculosis, MVA85A, induces high levels of cellular immune responses in UK volunteers. We assessed the safety and immunogenicity of this new vaccine in West African volunteers.
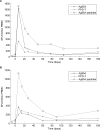
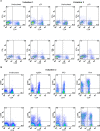
Methods and findings: We vaccinated 21 healthy adult male subjects (11 BCG scar negative and 10 BCG scar positive) with MVA85A after screening for evidence of prior exposure to mycobacteria. We monitored them over six months, observing for clinical, haematological and biochemical adverse events, together with assessment of the vaccine induced cellular immune response using ELISPOT and flow cytometry. MVA85A was well tolerated with no significant adverse events. Mild local and systemic adverse events were consistent with previous UK trials. Marked immunogenicity was found whether individuals had a previous BCG scar or not. There was not enhanced immunogenicity in those with a BCG scar, and induced T cell responses were better maintained in apparently BCG-naïve Gambians than previously studied BCG-naïve UK vaccinees. Although responses were predominantly attributable to CD4+ T cells, we also identified antigen specific CD8+ T cell responses, in subjects who were HLA B-35 and in whom enough blood was available for more detailed immunological analysis.
Conclusions: These data on the safety and immunogenicity of MVA85A in West Africa support its accelerated development as a promising booster vaccine for tuberculosis.
Trial registration: ClinicalTrials.gov NCT00423839.
Conflict of interest statement
Figures

References
-
- Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. - PubMed
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. - PubMed
-
- Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–1401. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

