Suppression of HIV-specific and allogeneic T cell activation by human regulatory T cells is dependent on the strength of signals
- PMID: 18698349
- PMCID: PMC2490715
- DOI: 10.1371/journal.pone.0002952
Suppression of HIV-specific and allogeneic T cell activation by human regulatory T cells is dependent on the strength of signals
Abstract
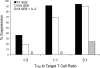
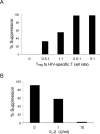
Regulatory T cells (Tregs) suppress immune responses against both self and non-self antigens. Tregs require activation through the T cell receptor (TCR) and IL-2 to exert their suppressive functions. However, how strength of TCR signals modulate the potency of Treg-mediated suppression of antigen-specific T cell activation remain unclear. We found that both strength of TCR signals and ratios of Tregs to target cells, either through superantigen, allogeneic antigens or HIV-specific peptides, modified the suppressive ability of Tregs. While human Tregs were able to mediate suppression in the presence of only autologous antigen-presenting cells, this was much less efficient as compared to when Tregs were activated by allogeneic dendritic cells. In another physiologically relevant system, we show that the strength of peptide stimulation, high frequency of responder CD8+ T cells or presence of high IL-2 can override the suppression of HIV-specific CD8+ T cells by Tregs. These findings suggest that ratios and TCR activation of human Tregs, are important parameters to overcome robust immune responses to pathogens or allogeneic antigens. Modulating the strength of T cell signals and selective enhancement or depletion of antigen-specific Tregs thus may have implications for designing potent vaccines and regulating immune responses during allogeneic transplantation and chronic infections.
Conflict of interest statement
Figures



References
-
- Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–544. - PubMed
-
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. - PubMed
-
- Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

