Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria
- PMID: 18698359
- PMCID: PMC2491586
- DOI: 10.1371/journal.pone.0002940
Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria
Abstract
Background: Apical Membrane Antigen 1 (AMA1), a polymorphic merozoite surface protein, is a leading blood-stage malaria vaccine candidate. This is the first reported use in humans of an investigational vaccine, AMA1-C1/Alhydrogel, with the novel adjuvant CPG 7909.
Methods: A phase 1 trial was conducted at the University of Rochester with 75 malaria-naive volunteers to assess the safety and immunogenicity of the AMA1-C1/Alhydrogel+CPG 7909 malaria vaccine. Participants were sequentially enrolled and randomized within dose escalating cohorts to receive three vaccinations on days 0, 28 and 56 of either 20 microg of AMA1-C1/Alhydrogel+564 microg CPG 7909 (n = 15), 80 microg of AMA1-C1/Alhydrogel (n = 30), or 80 microg of AMA1-C1/Alhydrogel+564 microg CPG 7909 (n = 30).
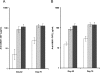
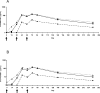
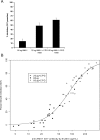
Results: Local and systemic adverse events were significantly more likely to be of higher severity with the addition of CPG 7909. Anti-AMA1 immunoglobulin G (IgG) were detected by enzyme-linked immunosorbent assay (ELISA), and the immune sera of volunteers that received 20 microg or 80 microg of AMA1-C1/Alhydrogel+CPG 7909 had up to 14 fold significant increases in anti-AMA1 antibody concentration compared to 80 microg of AMA1-C1/Alhydrogel alone. The addition of CPG 7909 to the AMA1-C1/Alhydrogel vaccine in humans also elicited AMA1 specific immune IgG that significantly and dramatically increased the in vitro growth inhibition of homologous parasites to levels as high as 96% inhibition.
Conclusion/significance: The safety profile of the AMA1-C1/Alhydrogel+CPG 7909 malaria vaccine is acceptable, given the significant increase in immunogenicity observed. Further clinical development is ongoing.
Trial registration: ClinicalTrials.gov NCT00344539.
Conflict of interest statement
Figures
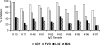
References
-
- WHO. World Malaria Report, World Health Organization. 2005.
-
- Anders RF, Crewther PE, Edwards S, Margetts M, Matthew ML, et al. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. - PubMed
-
- Deans JA, Knight AM, Jean WC, Waters AP, Cohen S, et al. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 1988;10:535–552. - PubMed
-
- Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, et al. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

