Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease
- PMID: 18698410
- PMCID: PMC2492828
- DOI: 10.1371/journal.pone.0002935
Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease
Abstract
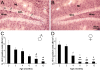
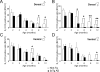
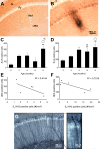
It has become generally accepted that new neurones are added and integrated mainly in two areas of the mammalian CNS, the subventricular zone and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus, which is of central importance in learning and memory. The newly generated cells display neuronal morphology, are able to generate action potentials and receive functional synaptic inputs, i.e. their properties are similar to those found in mature neurones. Alzheimer's disease (AD) is the primary and widespread cause of dementia and is an age-related, progressive and irreversible neurodegenerative disease that deteriorates cognitive functions. Here, we have used male and female triple transgenic mice (3xTg-AD) harbouring three mutant genes (beta-amyloid precursor protein, presenilin-1 and tau) and their respective non-transgenic (non-Tg) controls at 2, 3, 4, 6, 9 and 12 months of age to establish the link between AD and neurogenesis. Using immunohistochemistry we determined the area density of proliferating cells within the SGZ of the DG, measured by the presence of phosphorylated Histone H3 (HH3), and their possible co-localisation with GFAP to exclude a glial phenotype. Less than 1% of the HH3 labeled cells co-localised with GFAP. Both non-Tg and 3xTg-AD showed an age-dependent decrease in neurogenesis. However, male 3xTg-AD mice demonstrated a further reduction in the production of new neurones from 9 months of age (73% decrease) and a complete depletion at 12 months, when compared to controls. In addition, female 3xTg-AD mice showed an earlier but equivalent decrease in neurogenesis at 4 months (reduction of 63%) with an almost inexistent rate at 12 months (88% decrease) compared to controls. This reduction in neurogenesis was directly associated with the presence of beta-amyloid plaques and an increase in the number of beta-amyloid containing neurones in the hippocampus; which in the case of 3xgTg females was directly correlated. These results suggest that 3xTg-AD mice have an impaired ability to generate new neurones in the DG of the hippocampus, the severity of which increases with age and might be directly associated with the known cognitive impairment observed from 6 months of age onwards . The earlier reduction of neurogenesis in females, from 4 months, is in agreement with the higher prevalence of AD in women than in men. Thus it is conceivable to speculate that a recovery in neurogenesis rates in AD could help to rescue cognitive impairment.
Conflict of interest statement
Figures

References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Ramón y Cajal S. Madrid: Imprenta de Hijos de Nicolás Moya; 1913. Estudios sobre la degeneración y regeneración del sistema nervioso.
-
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. - PubMed
-
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. - PubMed
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

