Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization
- PMID: 18699862
- PMCID: PMC2692389
- DOI: 10.1111/j.1471-4159.2008.05617.x
Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization
Abstract
Cerebellar Purkinje cells (PC) are particularly vulnerable to ischemic injury and excitotoxicity, although the molecular basis of this sensitivity remains unclear. We tested the hypothesis that ischemia causes rapid down-regulation of GABA(A) receptors in cerebellar PC, thereby increasing susceptibility to excitotoxicity. Oxygen-glucose deprivation (OGD) caused a decline in functional GABA(A) receptors, within the first hour of re-oxygenation. Decreased amplitude of miniature inhibitory post-synaptic potentials confirmed that OGD caused a significant decrease in functional synaptic GABA(A) receptors and quantitative Western blot analysis demonstrated the loss of GABA(A) receptor current was associated with a decline in total receptor protein. Interestingly, the potent neuroprotectant allopregnanolone (ALLO) prevented the decline in GABA(A) receptor current and protein. Consistent with our in vitro data, global ischemia in mice caused a significant decline in total cerebellar GABA(A) receptor protein and PC specific immunoreactivity. Moreover, ALLO provided strong protection of PC and prevented ischemia-induced decline in GABA(A) receptor protein. Our findings indicate that ischemia causes a rapid and sustained loss of GABA(A) receptors in PC, whereas ALLO prevents the decline in GABA(A) receptors and protects against ischemia-induced damage. Thus, interventions which prevent ischemia-induced decline in GABA(A) receptors may represent a novel neuroprotective strategy.
Figures


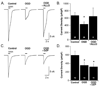

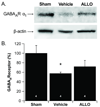

References
-
- Alicke B, Schwartz-Bloom RD. Rapid down-regulation of GABAA receptors in the gerbil hippocampus following transient cerebral ischemia. J. Neurochem. 1995;65:2808–2811. - PubMed
-
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6:565–575. - PubMed
-
- Brasko J, Rai P, Sabol MK, Patrikios P, Ross DT. The AMPA antagonist NBQX provides partial protection of rat cerebellar Purkinje cells after cardiac arrest and resuscitation. Brain Res. 1995;699:133–138. - PubMed
-
- Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

