The use of nonhuman primate models in HIV vaccine development
- PMID: 18700814
- PMCID: PMC2504486
- DOI: 10.1371/journal.pmed.0050173
The use of nonhuman primate models in HIV vaccine development
Abstract
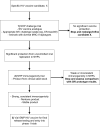
Cecilia Morgan and colleagues outline a two-stage nonhuman primate screening strategy for T cell-based HIV-1 vaccines.
Conflict of interest statement
Figures

References
-
- Desrosiers RC. Prospects for an AIDS vaccine. Nat Med. 2004;10:221–223. - PubMed
-
- Feinberg MB, Moore JP. AIDS vaccine models: Challenging challenge viruses. Nat Med. 2002;8:207–210. - PubMed
-
- Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev. 2004;6:187–198. - PubMed
-
- Johnson RP. HIV pathogenesis and vaccine development. Top HIV Med. 2006;14:8–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

