Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands
- PMID: 18700879
- PMCID: PMC2729103
- DOI: 10.1111/j.1399-0039.2008.01106.x
Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands
Abstract
The crucial role played by human leukocyte antigen (HLA) antigens and natural killer (NK)-cell-activating ligands in the interactions of malignant cells with components of the host's immune system has stimulated interest in the characterization of their expression by malignant cells. Convincing evidence generated by the immunohistochemical staining of surgically removed malignant lesions with monoclonal antibodies recognizing HLA antigens and NK-cell-activating ligands indicates that the surface expression of these molecules is frequently altered on malignant cells. These changes appear to have clinical significance because in some types of malignant disease they are associated with the histopathological characteristics of the lesions as well as with disease-free interval and survival. These associations have been suggested to reflect the effect of HLA antigen and NK-cell-activating ligand abnormalities on the interactions of tumor cells with antigen-specific cytotoxic T lymphocytes (CTL) and with NK cells. Nevertheless, there are examples in which disease progresses in the face of appropriate HLA antigen and/or NK-cell-activating ligand as well as tumor antigen expression by malignant cells and of functional antigen-specific CTL in the investigated patient. In such scenarios, it is likely that the tumor microenvironment is unfavorable for CTL and NK cell activity and contributes to tumor immune escape. Many distinct escape mechanisms have been shown to protect malignant cells from immune recognition and destruction in the tumor microenvironment. In this article, following the description of the structural and functional characteristics of soluble HLA antigens and NK-cell-activating ligands, we will review changes in their serum level in malignant disease and discuss their potential role in the escape mechanisms used by tumor cells to avoid recognition and destruction.
Figures
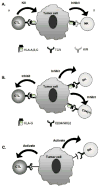

 ) are detectable in plasma as 43kDa, 39kDa and 35kDa moieties which represent membrane associated, alternatively spliced and metalloprotease cleaved forms, respectively. Splicing of exon 5 results in the removal of amino acids depicted in white (●), but not residues encoded by exons 6 and 7 (●). Metalloprotease mediated cleavage likely results in removal of amino acids encoded by exons 5–7. All three forms are shown to be associated with β2m (
) are detectable in plasma as 43kDa, 39kDa and 35kDa moieties which represent membrane associated, alternatively spliced and metalloprotease cleaved forms, respectively. Splicing of exon 5 results in the removal of amino acids depicted in white (●), but not residues encoded by exons 6 and 7 (●). Metalloprotease mediated cleavage likely results in removal of amino acids encoded by exons 5–7. All three forms are shown to be associated with β2m ( ) and peptide (
) and peptide ( ); however, β2m-free heavy chains have also been detected. (B) HLA-G is detectable in seven forms. Four of them, HLA-G1, -G2, -G3 and -G4, are bound to the cell surface, while the remaining three, HLA-G5, -G6 and -G7 are soluble. HLA-G1 is the only isoform derived from the translation of the total HLA-G transcript. The other membrane bound isoforms lack one or two globular domains. The structure of the soluble isoforms resembles that of the corresponding membrane bound isoforms in the extracellular part, but differs at the C-terminus. The extracellular domain and the intracytoplasmic tail, which are present in the membrane bound isoforms, are replaced in the secreted isoforms by a short hydrophilic tail. These differences provide a marker to distinguish shed or proteolytically cleaved HLA-G isoforms from secreted HLA-G isoforms.
); however, β2m-free heavy chains have also been detected. (B) HLA-G is detectable in seven forms. Four of them, HLA-G1, -G2, -G3 and -G4, are bound to the cell surface, while the remaining three, HLA-G5, -G6 and -G7 are soluble. HLA-G1 is the only isoform derived from the translation of the total HLA-G transcript. The other membrane bound isoforms lack one or two globular domains. The structure of the soluble isoforms resembles that of the corresponding membrane bound isoforms in the extracellular part, but differs at the C-terminus. The extracellular domain and the intracytoplasmic tail, which are present in the membrane bound isoforms, are replaced in the secreted isoforms by a short hydrophilic tail. These differences provide a marker to distinguish shed or proteolytically cleaved HLA-G isoforms from secreted HLA-G isoforms.
Similar articles
-
Enhancing Natural Killer and CD8+ T Cell-Mediated Anticancer Cytotoxicity and Proliferation of CD8+ T Cells with HLA-E Monospecific Monoclonal Antibodies.Monoclon Antib Immunodiagn Immunother. 2019 Apr;38(2):38-59. doi: 10.1089/mab.2018.0043. Monoclon Antib Immunodiagn Immunother. 2019. PMID: 31009335 Free PMC article. Review.
-
NK cell activating ligands on human malignant cells: molecular and functional defects and potential clinical relevance.Semin Cancer Biol. 2006 Oct;16(5):383-92. doi: 10.1016/j.semcancer.2006.07.001. Epub 2006 Jul 7. Semin Cancer Biol. 2006. PMID: 16931041 Review.
-
Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions.Adv Cancer Res. 2005;93:189-234. doi: 10.1016/S0065-230X(05)93006-6. Adv Cancer Res. 2005. PMID: 15797448 Review.
-
Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation.Eur J Immunol. 2003 Jan;33(1):125-34. doi: 10.1002/immu.200390015. Eur J Immunol. 2003. PMID: 12594841
-
Re-targeting of cytotoxic T lymphocytes and/or natural killer cells to CEA-expressing tumor cells with anti-CEA antibody activity.Anticancer Res. 2005 Nov-Dec;25(6A):3725-32. Anticancer Res. 2005. PMID: 16302732 Review.
Cited by
-
Therapeutic applications of the selective high affinity ligand drug SH7139 extend beyond non-Hodgkin's lymphoma to many other types of solid cancers.Oncotarget. 2020 Sep 1;11(35):3315-3349. doi: 10.18632/oncotarget.27709. eCollection 2020 Sep 1. Oncotarget. 2020. PMID: 32934776 Free PMC article.
-
Cancer Immunoediting: Elimination, Equilibrium, and Immune Escape in Solid Tumors.Exp Suppl. 2022;113:1-57. doi: 10.1007/978-3-030-91311-3_1. Exp Suppl. 2022. PMID: 35165859
-
Soluble HLA in the Aqueous Humour of Uveal Melanoma Is Associated with Unfavourable Tumour Characteristics.Cancers (Basel). 2019 Aug 18;11(8):1202. doi: 10.3390/cancers11081202. Cancers (Basel). 2019. PMID: 31426578 Free PMC article.
-
Identification of Tumor Antigens Among the HLA Peptidomes of Glioblastoma Tumors and Plasma.Mol Cell Proteomics. 2019 Jun;18(6):1255-1268. doi: 10.1074/mcp.RA119.001524. Mol Cell Proteomics. 2019. PMID: 31154438 Free PMC article.
-
Cancer stem cell immunology: key to understanding tumorigenesis and tumor immune escape?Front Immunol. 2014 Jul 29;5:360. doi: 10.3389/fimmu.2014.00360. eCollection 2014. Front Immunol. 2014. PMID: 25120546 Free PMC article. Review.
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
-
- Marchand M, Weynants P, Rankin E, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883–885. - PubMed
-
- Jaeger E, Bernahrd H, Romero P, Ringhoffer M, Arand M, Karbach J, Ilsemann C, Hagedorn M, Knuth A. Generation of cytotoxic T-cell responses with synthetic peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer. 1996;66:162–169. - PubMed
-
- Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805–818. - PubMed
-
- Durrant LG, Spendlove I. Cancer vaccines entering Phase III clinical trials. Expert Opin Emerg Drugs. 2003;8:489–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

