Modification of solid phase red cell adherence assay for the detection of platelet antibodies in patients with thrombocytopenia
- PMID: 18701420
- PMCID: PMC2628744
- DOI: 10.1309/1QWTQFMF0Q9JEAGR
Modification of solid phase red cell adherence assay for the detection of platelet antibodies in patients with thrombocytopenia
Abstract
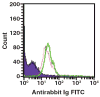
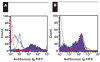
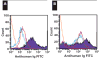
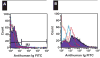
Platelet refractoriness is caused by HLA antibodies and platelet-specific antibodies. Current methods used to detect antiplatelet antibodies have limitations. Solid phase red cell adherence (SPRCA) lacks sensitivity and requires a second assay using chloroquine-treated intact platelets to specify the response due to anti-HLA. We modified SPRCA by using 2 types of antihuman platelet antibodies with different specificities toward platelet lysate and tested samples from 361 patients (69 with unexplained thrombocytopenia and 292 with poor response to platelet transfusions not explicable by alloimmunization or the clinical situation) and 50 from healthy volunteers. Our method compared favorably with platelet suspension direct immunofluorescence. All samples from healthy volunteers were negative; of the samples from the patient population, 240 were positive (147 samples had only antiplatelet and 3 samples had only anti-HLA antibodies). This modified technique had a sensitivity of 98% and a specificity of 91%.
Figures






Similar articles
-
Use of enzyme treatment to enhance reactivity of HLA and platelet-specific antibodies in solid-phase RBC adherence assays.Transfusion. 2002 Apr;42(4):476-80. doi: 10.1046/j.1525-1438.2002.00083.x. Transfusion. 2002. PMID: 12076296
-
Human Leukocyte Antigen Alloimmunization and Alloimmune Platelet Refractoriness.Transfus Med Rev. 2020 Oct;34(4):250-257. doi: 10.1016/j.tmrv.2020.09.010. Epub 2020 Oct 7. Transfus Med Rev. 2020. PMID: 33127210 Review.
-
Application of lyophilised human platelets for antibody detection in solid phase red cell adherence assay.J Immunol Methods. 2020 Dec;487:112868. doi: 10.1016/j.jim.2020.112868. Epub 2020 Sep 15. J Immunol Methods. 2020. PMID: 32941887
-
Evaluation of a solid phase red cell adherence technique for platelet antibody screening.Transfus Med. 1991 Sep;1(3):163-7. doi: 10.1111/j.1365-3148.1991.tb00026.x. Transfus Med. 1991. PMID: 9259843
-
[Platelet allo-antibodies identification strategies for preventing and managing platelet refractoriness].Transfus Clin Biol. 2014 Nov;21(4-5):193-206. doi: 10.1016/j.tracli.2014.08.140. Epub 2014 Sep 30. Transfus Clin Biol. 2014. PMID: 25277423 Review. French.
Cited by
-
Evaluation of platelet cross-matching in the management of patients refractory to platelet transfusions.Blood Transfus. 2014 Apr;12(2):187-94. doi: 10.2450/2014.0120-13. Blood Transfus. 2014. PMID: 24931840 Free PMC article. Clinical Trial.
References
-
- Slichter SJ. Platelet transfusions a constantly evolving therapy. Thromb Haemost. 1991;66:178–188. - PubMed
-
- Herman JH, Kamel HT. Platelet transfusion: current techniques, remaining problems, and future prospects. Am J Pediatr Hematol Oncol. 1987;9:272–286. - PubMed
-
- Kiefel V, Konig C, Kroll H, et al. Platelet alloantibodies in transfused patients. Transfusion. 2001;41:766–770. - PubMed
-
- Buetens O, Shirey RS, Goble-Lee M, et al. Prevalence of HLA antibodies in transfused patients with and without red cell antibodies. Transfusion. 2006;46:754–756. - PubMed
-
- Bajpai M, Kaura B, Marwaha N, et al. Platelet alloimmunization in multitransfused patients with haemato-oncological disorders. Natl Med J India. 2005;18:134–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

