CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail
- PMID: 18701457
- PMCID: PMC2662074
- DOI: 10.1074/jbc.M804613200
CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail
Abstract
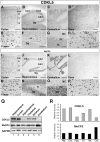
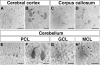
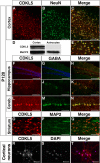
Mutations in the human X-linked cyclin-dependent kinase-like 5 (CDKL5) gene have been identified in patients with Rett syndrome (RTT), West syndrome, and X-linked infantile spasms, sharing the common feature of mental retardation and early seizures. CDKL5 is a rather uncharacterized kinase, but its involvement in RTT seems to be explained by the fact that it works upstream of MeCP2, the main cause of Rett syndrome. To understand the role of this kinase for nervous system functions and to address if molecular mechanisms are involved in regulating its distribution and activity, we studied the ontogeny of CDKL5 expression in developing mouse brains by immunostaining and Western blotting. The expression profile of CDKL5 was compared with that of MeCP2. The two proteins share a general expression profile in the adult mouse brain, but CDKL5 levels appear to be highly modulated at the regional level. Its expression is strongly induced in early postnatal stages, and in the adult brain CDKL5 is present in mature neurons, but not in astroglia. Interestingly, the presence of CDKL5 in the cell nucleus varies at the regional level of the adult brain and is developmentally regulated. CDKL5 shuttles between the cytoplasm and the nucleus and the C-terminal tail is involved in localizing the protein to the cytoplasm in a mechanism depending on active nuclear export. Accordingly, Rett derivatives containing disease-causing truncations of the C terminus are constitutively nuclear, suggesting that they might act as gain of function mutations in this cellular compartment.
Figures




References
-
- Hagberg, B., Aicardi, J., Dias, K., and Ramos, O. (1983) Ann. Neurol. 14 471-479 - PubMed
-
- Hagberg, B., Goutieres, F., Hanefeld, F., Rett, A., and Wilson, J. (1985) Brain Dev. 7 372-373 - PubMed
-
- Armstrong, D. D. (2002) Ment. Retard. Dev. Disabil. Res. Rev. 8 72-76 - PubMed
-
- Hagberg, B. A., and Skjeldal, O. H. (1994) Pediatr. Neurol. 11 5-11 - PubMed
-
- Amir, R. E. (1999) Nat. Genet. 23 185-188 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

