A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin
- PMID: 18701497
- PMCID: PMC3767150
- DOI: 10.1158/0008-5472.CAN-08-1123
A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin
Abstract
A novel acetylenic tricyclic bis-(cyano enone), TBE-31, is a lead compound in a series of tricyclic compounds with enone functionalities in rings A and C. Nanomolar concentrations of this potent multifunctional molecule suppress the induction of the inflammatory protein, inducible nitric oxide synthase, activate phase 2 cytoprotective enzymes in vitro and in vivo, block cell proliferation, and induce differentiation and apoptosis of leukemia cells. Oral administration of TBE-31 also significantly reduces formation of aflatoxin-DNA adducts and decreases size and number of aflatoxin-induced preneoplastic hepatic lesions in rats by >90%. Because of the two cyano enones in rings A and C, TBE-31 may directly interact with DTT and protein targets such as Keap1 that contain reactive cysteine residues. The above findings suggest that TBE-31 should also be tested for chemoprevention and chemotherapy in relevant models of cancer and against other chronic, degenerative diseases in which inflammation and oxidative stress contribute to disease pathogenesis.
Figures


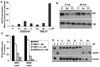
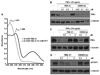


Similar articles
-
Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents.J Med Chem. 2011 Mar 24;54(6):1762-78. doi: 10.1021/jm101445p. Epub 2011 Mar 1. J Med Chem. 2011. PMID: 21361338 Free PMC article.
-
Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole.Cancer Res. 2006 Feb 15;66(4):2488-94. doi: 10.1158/0008-5472.CAN-05-3823. Cancer Res. 2006. PMID: 16489057
-
Efficient synthesis of (-)- and (+)-tricyclic compounds with enone functionalities in rings A and C. A novel class of orally active anti-inflammatory and cancer chemopreventive agents.Org Biomol Chem. 2003 Dec 21;1(24):4384-91. doi: 10.1039/b307491a. Epub 2003 Oct 31. Org Biomol Chem. 2003. PMID: 14685310
-
[New paradigm of host defense against intracellular pathogens by nitric oxide].Nihon Hansenbyo Gakkai Zasshi. 2009 Feb;78(1):41-7. doi: 10.5025/hansen.78.41. Nihon Hansenbyo Gakkai Zasshi. 2009. PMID: 19227148 Review. Japanese.
-
Role of phase 2 enzyme induction in chemoprotection by dithiolethiones.Mutat Res. 2001 Sep 1;480-481:305-15. doi: 10.1016/s0027-5107(01)00190-7. Mutat Res. 2001. PMID: 11506823 Review.
Cited by
-
Aldo-keto reductases are biomarkers of NRF2 activity and are co-ordinately overexpressed in non-small cell lung cancer.Br J Cancer. 2016 Dec 6;115(12):1530-1539. doi: 10.1038/bjc.2016.363. Epub 2016 Nov 8. Br J Cancer. 2016. PMID: 27824809 Free PMC article.
-
The Role of Sulfhydryl Reactivity of Small Molecules for the Activation of the KEAP1/NRF2 Pathway and the Heat Shock Response.Scientifica (Cairo). 2012;2012:606104. doi: 10.6064/2012/606104. Epub 2012 Dec 23. Scientifica (Cairo). 2012. PMID: 24278719 Free PMC article. Review.
-
Nrf2 activation does not affect adenoma development in a mouse model of colorectal cancer.Commun Biol. 2021 Sep 15;4(1):1081. doi: 10.1038/s42003-021-02552-w. Commun Biol. 2021. PMID: 34526660 Free PMC article.
-
Oral administration of a gemini vitamin D analog, a synthetic triterpenoid and the combination prevents mammary tumorigenesis driven by ErbB2 overexpression.Cancer Prev Res (Phila). 2013 Sep;6(9):959-70. doi: 10.1158/1940-6207.CAPR-13-0087. Epub 2013 Jul 15. Cancer Prev Res (Phila). 2013. PMID: 23856074 Free PMC article.
-
Lactone metabolite common to the carcinogens dioxane, diethylene glycol, and N-nitrosomorpholine: aqueous chemistry and failure to mediate liver carcinogenesis in the F344 rat.Chem Res Toxicol. 2012 May 21;25(5):1022-8. doi: 10.1021/tx3000076. Epub 2012 Apr 12. Chem Res Toxicol. 2012. PMID: 22458541 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. - PubMed
-
- Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. - PubMed
-
- Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392–2404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

