Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model
- PMID: 18701508
- PMCID: PMC2587411
- DOI: 10.1158/0008-5472.CAN-08-1332
Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model
Abstract
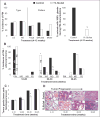
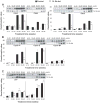
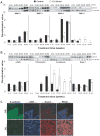
Herein, using transgenic adenocarcinoma of the mouse prostate (TRAMP) model, we assessed the "stage-specific" efficacy of silibinin feeding against prostate cancer (PCa) initiation, progression, angiogenesis and metastasis, and associated molecular events involved in silibinin effects during these stages. Male TRAMP mice starting at ages 4, 12, 20, and 30 weeks of age were fed with control or 1% silibinin-supplemented diet for 8 to 15 weeks in stage-specific manners. At the end of studies, silibinin-fed mice showed less severe prostatic lesions compared with positive controls. During early stages of prostate tumor development, silibinin mediated its efficacy mostly via antiproliferative mechanisms. Feeding of silibinin to animals burdened with higher stages of prostate tumor significantly decreased tumor grade via antiproliferative effect, and inhibition of angiogenesis as evidenced by decreased expressions of platelet endothelial cell adhesion molecule-1/CD-31, vascular endothelial growth factor, and associated receptor, vascular endothelial growth factor R2, hypoxia-inducible factor-1alpha, and inducible nitric oxide synthase. Metastasis to distant organs was decreased in silibinin-fed mice, which was associated with a decreased expression of matrix metalloproteinases, mesenchymal markers snail-1, and fibronectin in the prostatic tissue and retention of epithelial characteristics. Together, these findings are both novel and highly significant in establishing the dual efficacy of silibinin where it inhibits progression of primary prostatic tumor and also shows protective efficacy against angiogenesis and late stage metastasis. These effects of silibinin could have potential implications to improve the morbidity and survival in PCa patients.
Figures


Similar articles
-
Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice.Clin Cancer Res. 2008 Dec 1;14(23):7773-80. doi: 10.1158/1078-0432.CCR-08-1309. Clin Cancer Res. 2008. PMID: 19047104 Free PMC article.
-
Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model.Cancer Res. 2007 Nov 15;67(22):11083-91. doi: 10.1158/0008-5472.CAN-07-2222. Cancer Res. 2007. PMID: 18006855
-
Effect of silibinin on the growth and progression of primary lung tumors in mice.J Natl Cancer Inst. 2006 Jun 21;98(12):846-55. doi: 10.1093/jnci/djj231. J Natl Cancer Inst. 2006. PMID: 16788158
-
Prostate cancer prevention by silibinin.Curr Cancer Drug Targets. 2004 Feb;4(1):1-11. doi: 10.2174/1568009043481605. Curr Cancer Drug Targets. 2004. PMID: 14965263 Review.
-
Prostate cancer chemoprevention by silibinin: bench to bedside.Mol Carcinog. 2006 Jun;45(6):436-42. doi: 10.1002/mc.20223. Mol Carcinog. 2006. PMID: 16637061 Review.
Cited by
-
Co-Targeting Tumor Angiogenesis and Immunosuppressive Tumor Microenvironment: A Perspective in Ethnopharmacology.Front Pharmacol. 2022 Jun 15;13:886198. doi: 10.3389/fphar.2022.886198. eCollection 2022. Front Pharmacol. 2022. PMID: 35784750 Free PMC article. Review.
-
Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity.Sci Rep. 2016 Mar 16;6:23135. doi: 10.1038/srep23135. Sci Rep. 2016. PMID: 26979487 Free PMC article.
-
Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy.Curr Cancer Drug Targets. 2013 Jun;13(5):486-99. doi: 10.2174/15680096113139990041. Curr Cancer Drug Targets. 2013. PMID: 23617249 Free PMC article. Review.
-
Deletion of p21/Cdkn1a confers protective effect against prostate tumorigenesis in transgenic adenocarcinoma of the mouse prostate model.Cell Cycle. 2013 May 15;12(10):1598-604. doi: 10.4161/cc.24741. Epub 2013 Apr 25. Cell Cycle. 2013. PMID: 23624841 Free PMC article.
-
The Radiosensitizing Potentials of Silymarin/Silibinin in Cancer: A Systematic Review.Curr Med Chem. 2024;31(42):6992-7014. doi: 10.2174/0109298673248404231006052436. Curr Med Chem. 2024. PMID: 37921180
References
-
- Stewart AB, Lwaleed BA, Douglas DA, Birch BR. Current drug therapy for prostate cancer: an overview. Curr Med Chem Anticancer Agents. 2005;5:603–12. - PubMed
-
- Brand TC, Canby-Hagino ED, Pratap Kumar A, et al. Chemoprevention of prostate cancer. Hematol Oncol Clin North Am. 2006;20:831–43. - PubMed
-
- Klein EA. Chemoprevention of prostate cancer. Annu Rev Med. 2006;57:49–63. - PubMed
-
- Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–78. - PubMed
-
- Thompson IM. Chemoprevention of prostate cancer: agents and study designs. J Urol. 2007;178:S9–S13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

