Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial
- PMID: 18701556
- PMCID: PMC2674550
- DOI: 10.1136/ard.2007.087270
Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial
Abstract
Objective: To determine the long-term effect of adalimumab on patients with ankylosing spondylitis (AS) who participated in the Adalimumab Trial Evaluating Long-Term Efficacy and Safety in AS (ATLAS), a randomised, double-blind, placebo controlled, 24-week trial.
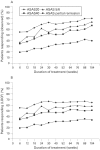
Methods: Patients received adalimumab 40 mg every other week (eow) or placebo for 24 weeks in ATLAS. At week 24, patients were switched to open-label adalimumab 40 mg eow. Efficacy measures included 20% improvement in the Assessment in SpondyloArthritis International Society (ASAS) criteria (ASAS20), ASAS40 and ASAS partial remission responses and changes in individual components of the ASAS20 response evaluations, for example, Bath AS Functional Index (BASFI) and Bath AS Disease Activity Index (BASDAI). Two-year interim data were analysed based on the total duration of adalimumab exposure, irrespective of the treatment randomisation group.
Results: At 2 years, 255 (82.0%) of the original 311 ATLAS patients continued receiving adalimumab treatment. Improvements in ASAS responses observed in ATLAS were sustained during long-term treatment; 64.5% (200/310) were ASAS20 responders, 50.6% (157/310) were ASAS40 responders and 33.5% (104/310) had maintained ASAS-defined partial remission. Changes in individual ASAS response components were sustained or improved during long-term adalimumab treatment. From ATLAS baseline to 2 years of adalimumab exposure, respectively, BASDAI improved from 6.3 (SD 1.7) to 2.4 (SD 2.3) and BASFI improved from 5.2 (SD 2.4) to 2.9 (SD 2.5). Adalimumab was well tolerated. No cases of tuberculosis, congestive heart failure, lupus-like symptoms, or demyelinating disease were reported.
Conclusions: Adalimumab reduced the signs and symptoms of AS and induced partial remission for up to 2 years. The long-term safety profile was similar to the short-term safety profile. Trial registration information: NCT00085644.
Conflict of interest statement
Figures
References
-
- Akkoc N, Khan MA. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al [letter]. Arthritis Rheum 2005;52:4048–9 - PubMed
-
- Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol 2006;20:401–17 - PubMed
-
- Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol 2006;33(Suppl 78):4–11 - PubMed
-
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93 - PubMed
-
- Braun J, Landewe R, Hermann KG, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials