The vaccinia virus gene I2L encodes a membrane protein with an essential role in virion entry
- PMID: 18701587
- PMCID: PMC2566298
- DOI: 10.1128/JVI.01035-08
The vaccinia virus gene I2L encodes a membrane protein with an essential role in virion entry
Abstract
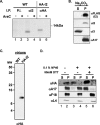
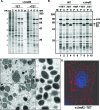
The previously unstudied vaccinia virus gene I2L is conserved in all orthopoxviruses. We show here that the 8-kDa I2 protein is expressed at late times of infection, is tightly associated with membranes, and is encapsidated in mature virions. We have generated a recombinant virus in which I2 expression is dependent upon the inclusion of tetracycline in the culture medium. In the absence of I2, the biochemical events of the viral life cycle progress normally, and virion morphogenesis culminates in the production of mature virions. However, these virions show an approximately 400-fold reduction in specific infectivity due to an inability to enter target cells. Several proteins that have been previously identified as components of an essential entry/fusion complex are present at reduced levels in I2-deficient virions, although other membrane proteins, core proteins, and DNA are encapsidated at normal levels. A preliminary structure/function analysis of I2 has been performed using a transient complementation assay: the C-terminal hydrophobic domain is essential for protein stability, and several regions within the N-terminal hydrophilic domain are essential for biological competency. I2 is thus yet another component of the poxvirus virion that is essential for the complex process of entry into target cells.
Figures

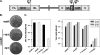




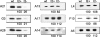
Similar articles
-
Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway.J Virol. 2004 Mar;78(5):2348-56. doi: 10.1128/jvi.78.5.2348-2356.2004. J Virol. 2004. PMID: 14963131 Free PMC article.
-
The vaccinia virus I1 protein is essential for the assembly of mature virions.J Virol. 1997 Dec;71(12):9285-94. doi: 10.1128/JVI.71.12.9285-9294.1997. J Virol. 1997. PMID: 9371587 Free PMC article.
-
Vaccinia virus morphogenesis: a13 phosphoprotein is required for assembly of mature virions.J Virol. 2004 Aug;78(16):8885-901. doi: 10.1128/JVI.78.16.8885-8901.2004. J Virol. 2004. PMID: 15280497 Free PMC article.
-
In a nutshell: structure and assembly of the vaccinia virion.Adv Virus Res. 2006;66:31-124. doi: 10.1016/S0065-3527(06)66002-8. Adv Virus Res. 2006. PMID: 16877059 Review.
-
Vaccinia virus morphogenesis and dissemination.Trends Microbiol. 2008 Oct;16(10):472-9. doi: 10.1016/j.tim.2008.07.009. Epub 2008 Sep 12. Trends Microbiol. 2008. PMID: 18789694 Review.
Cited by
-
Integrin β1 mediates vaccinia virus entry through activation of PI3K/Akt signaling.J Virol. 2012 Jun;86(12):6677-87. doi: 10.1128/JVI.06860-11. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496232 Free PMC article.
-
Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH.J Virol. 2012 Apr;86(7):3809-18. doi: 10.1128/JVI.06081-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278246 Free PMC article.
-
A turn-like structure "KKPE" segment mediates the specific binding of viral protein A27 to heparin and heparan sulfate on cell surfaces.J Biol Chem. 2009 Dec 25;284(52):36535-36546. doi: 10.1074/jbc.M109.037267. Epub 2009 Oct 26. J Biol Chem. 2009. PMID: 19858217 Free PMC article.
-
Role of the vaccinia virus O3 protein in cell entry can be fulfilled by its Sequence flexible transmembrane domain.Virology. 2013 Sep;444(1-2):148-57. doi: 10.1016/j.virol.2013.06.003. Epub 2013 Jun 29. Virology. 2013. PMID: 23816434 Free PMC article.
-
Inhibition of Vaccinia virus entry by a broad spectrum antiviral peptide.Virology. 2009 Jun 5;388(2):248-59. doi: 10.1016/j.virol.2009.03.023. Epub 2009 Apr 22. Virology. 2009. PMID: 19395056 Free PMC article.
References
-
- Bayliss, C. D., and R. C. Condit. 1995. The vaccinia virus A18R gene product is a DNA-dependent ATPase. J. Biol. Chem. 2701550-1556. - PubMed
-
- Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 861279-1290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

