Monomeric linear RNA of citrus exocortis viroid resulting from processing in vivo has 5'-phosphomonoester and 3'-hydroxyl termini: implications for the RNase and RNA ligase involved in replication
- PMID: 18701598
- PMCID: PMC2566280
- DOI: 10.1128/JVI.01229-08
Monomeric linear RNA of citrus exocortis viroid resulting from processing in vivo has 5'-phosphomonoester and 3'-hydroxyl termini: implications for the RNase and RNA ligase involved in replication
Abstract
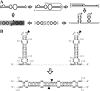
Members of the family Pospiviroidae, like Citrus exocortis viroid (CEVd), replicate through an RNA-based asymmetric rolling-circle mechanism in which oligomeric plus-strand [(+)] RNA intermediates are cleaved to monomeric linear (ml) RNA and then circularized. Here we show, by rapid amplification of 5' and 3' cDNA ends and in vitro ligation assays, that ml CEVd (+) RNA resulting from cleavage of a dimeric transcript transgenically expressed in Arabidopsis thaliana contains 5'-phosphomonoester and 3'-hydroxyl termini. The nature of these termini and the double-stranded structure previously proposed as the substrate for cleavage in vivo suggest that a type III RNase catalyzes cleavage and an RNA ligase distinct from tRNA ligase promotes circularization.
Figures

Similar articles
-
Processing of nuclear viroids in vivo: an interplay between RNA conformations.PLoS Pathog. 2007 Nov;3(11):e182. doi: 10.1371/journal.ppat.0030182. PLoS Pathog. 2007. PMID: 18052530 Free PMC article.
-
Involvement of the chloroplastic isoform of tRNA ligase in the replication of viroids belonging to the family Avsunviroidae.J Virol. 2012 Aug;86(15):8269-76. doi: 10.1128/JVI.00629-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623792 Free PMC article.
-
Arabidopsis thaliana has the enzymatic machinery for replicating representative viroid species of the family Pospiviroidae.Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6792-7. doi: 10.1073/pnas.0401090101. Epub 2004 Apr 19. Proc Natl Acad Sci U S A. 2004. PMID: 15096616 Free PMC article.
-
Current view and perspectives in viroid replication.Curr Opin Virol. 2021 Apr;47:32-37. doi: 10.1016/j.coviro.2020.12.004. Epub 2021 Jan 15. Curr Opin Virol. 2021. PMID: 33460914 Free PMC article. Review.
-
Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme.RNA Biol. 2011 Mar-Apr;8(2):200-6. doi: 10.4161/rna.8.2.14238. Epub 2011 Mar 1. RNA Biol. 2011. PMID: 21358283 Review.
Cited by
-
Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13805-10. doi: 10.1073/pnas.1206187109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869737 Free PMC article.
-
Circular RNAs as Therapeutic Agents and Targets.Front Physiol. 2018 Oct 9;9:1262. doi: 10.3389/fphys.2018.01262. eCollection 2018. Front Physiol. 2018. PMID: 30356745 Free PMC article. Review.
-
Viroid replication: rolling-circles, enzymes and ribozymes.Viruses. 2009 Sep;1(2):317-34. doi: 10.3390/v1020317. Epub 2009 Sep 14. Viruses. 2009. PMID: 21994552 Free PMC article.
-
Viroids: from genotype to phenotype just relying on RNA sequence and structural motifs.Front Microbiol. 2012 Jun 18;3:217. doi: 10.3389/fmicb.2012.00217. eCollection 2012. Front Microbiol. 2012. PMID: 22719735 Free PMC article.
-
RNA conformational changes in the life cycles of RNA viruses, viroids, and virus-associated RNAs.Biochim Biophys Acta. 2009 Sep-Oct;1789(9-10):571-83. doi: 10.1016/j.bbagrm.2009.05.005. Epub 2009 Jun 6. Biochim Biophys Acta. 2009. PMID: 19501200 Free PMC article. Review.
References
-
- Branch, A. D., and H. D. Robertson. 1984. A replication cycle for viroids and other small infectious RNAs. Science 223450-455. - PubMed
-
- Branch, A. D., H. D. Robertson, C. Greer, P. Gegenheimer, C. Peebles, and J. Abelson. 1982. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat-germ. Science 2171147-1149. - PubMed
-
- Comella, P., F. Pontvianne, S. Lahmy, F. Vignols, N. Barbezier, A. DeBures, E. Jobet, E. Brugidou, M. Echeverria, and J. Sáez-Vásquez. 2008. Characterization of a ribonuclease III-like protein required for cleavage of the pre-rRNA in the 3′ETS in Arabidopsis. Nucleic Acids Res. 361163-1175. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

