Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel
- PMID: 18701628
- PMCID: PMC2576154
- DOI: 10.1152/ajprenal.00440.2007
Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel
Abstract
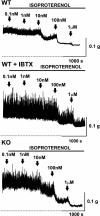
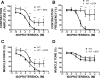
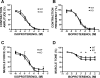
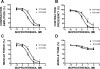
In urinary bladder smooth muscle (UBSM), stimulation of beta-adrenergic receptors (beta-ARs) leads to activation of the large-conductance Ca2+-activated K+ (BK) channel currents (Petkov GV and Nelson MT. Am J Physiol Cell Physiol 288: C1255-C1263, 2005). In this study we tested the hypothesis that the BK channel mediates UBSM relaxation in response to beta-AR stimulation using the highly specific BK channel inhibitor iberiotoxin (IBTX) and a BK channel knockout (BK-KO) mouse model in which the gene for the pore-forming subunit was deleted. UBSM strips isolated from wild-type (WT) and BK-KO mice were stimulated with 20 mM K+ or 1 microM carbachol to induce phasic and tonic contractions. BK-KO and WT UBSM strips pretreated with IBTX had increased overall contractility, and UBSM BK-KO cells were depolarized with approximately 12 mV. Isoproterenol, a nonspecific beta-AR agonist, and forskolin, an adenylate cyclase activator, decreased phasic and tonic contractions of WT UBSM strips in a concentration-dependent manner. In the presence of IBTX, the concentration-response curves to isoproterenol and forskolin were shifted to the right in WT UBSM strips. Isoproterenol- and forskolin-mediated relaxations were enhanced in BK-KO UBSM strips, and a leftward shift in the concentration-response curves was observed. The leftward shift was eliminated upon PKA inhibition with H-89, suggesting upregulation of the beta-AR-cAMP pathway in BK-KO mice. These results indicate that the BK channel is a key modulator in beta-AR-mediated relaxation of UBSM and further suggest that alterations in BK channel expression or function could contribute to some pathophysiological conditions such as overactive bladder and urinary incontinence.
Figures




References
-
- Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. - PubMed
-
- Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, Melman A. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281: R1699–R1709, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

