A lipid-protein hybrid model for tight junction
- PMID: 18701633
- PMCID: PMC2604825
- DOI: 10.1152/ajprenal.00097.2008
A lipid-protein hybrid model for tight junction
Abstract
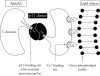
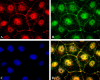
The epithelial tight junction (TJ) was first described ultrastructurally as a fusion of the outer lipid leaflets of the adjoining cell membrane bilayers (hemifusion). The discovery of an increasing number of integral TJ and TJ-associated proteins has eclipsed the original lipid-based model with the wide acceptance of a protein-centric model for the TJ. In this review, we stress the importance of lipids in TJ structure and function. A lipid-protein hybrid model accommodates a large body of information supporting the lipidic characteristics of the TJ, harmonizes with the accumulating evidence supporting the TJ as an assembly of lipid rafts, and focuses on an important, but relatively unexplored, field of lipid-protein interactions in the morphology, physiology, and pathophysiology of the TJ.
Figures




References
-
- Anderson JM Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci 16: 126–130, 2001. - PubMed
-
- Ayala-Sanmartin J, Henry JP, Pradel LA. Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca2+ concentration. Biochim Biophys Acta 1510: 18–28, 2001. - PubMed
-
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. - PMC - PubMed
-
- Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol 287: C327–C335, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

