Stimulation of glucose transport in response to activation of distinct AMPK signaling pathways
- PMID: 18701654
- PMCID: PMC2584987
- DOI: 10.1152/ajpcell.00040.2008
Stimulation of glucose transport in response to activation of distinct AMPK signaling pathways
Abstract
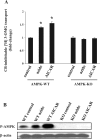
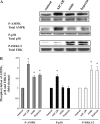
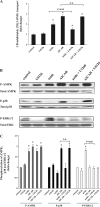
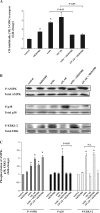
AMP-activated protein kinase (AMPK) plays a critical role in the stimulation of glucose transport in response to hypoxia and inhibition of oxidative phosphorylation. In the present study, we examined the signaling pathway(s) mediating the glucose transport response following activation of AMPK. Using mouse fibroblasts of AMPK wild type and AMPK knockout, we documented that the expression of AMPK is essential for the glucose transport response to both azide and 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR). In Clone 9 cells, the stimulation of glucose transport by a combination of azide and AICAR was not additive, whereas there was an additive increase in the abundance of phosphorylated AMPK (p-AMPK). In Clone 9 cells, AMPK wild-type fibroblasts, and H9c2 heart cells, azide or hypoxia selectively increased p-ERK1/2, whereas, in contrast, AICAR selectively stimulated p-p38; phosphorylation of JNK was unaffected. Azide's effect on p-ERK1/2 abundance and glucose transport in Clone 9 cells was partially abolished by the MEK1/2 inhibitor U0126. SB 203580, an inhibitor of p38, prevented the phosphorylation of p38 and the glucose transport response to AICAR and, unexpectedly, to azide. Hypoxia, azide, and AICAR all led to increased phosphorylation of Akt substrate of 160 kDa (AS160) in Clone 9 cells. Employing small interference RNA directed against AS160 did not inhibit the glucose transport response to azide or AICAR, whereas the content of P-AS160 was reduced by approximately 80%. Finally, we found no evidence for coimmunoprecipitation of Glut1 and p-AS160. We conclude that although azide, hypoxia, and AICAR all activate AMPK, the downstream signaling pathways are distinct, with azide and hypoxia stimulating ERK1/2 and AICAR stimulating the p38 pathway.
Figures





Similar articles
-
Critical role of 5'-AMP-activated protein kinase in the stimulation of glucose transport in response to inhibition of oxidative phosphorylation.Am J Physiol Cell Physiol. 2007 Jan;292(1):C477-87. doi: 10.1152/ajpcell.00196.2006. Epub 2006 Aug 30. Am J Physiol Cell Physiol. 2007. PMID: 16943243
-
Disruption of AMPKalpha1 signaling prevents AICAR-induced inhibition of AS160/TBC1D4 phosphorylation and glucose uptake in primary rat adipocytes.Mol Endocrinol. 2010 Jul;24(7):1434-40. doi: 10.1210/me.2009-0502. Epub 2010 May 25. Mol Endocrinol. 2010. PMID: 20501641 Free PMC article.
-
The effect of 5-aminoimidazole-4-carboxamide-ribonucleoside was mediated by p38 mitogen activated protein kinase signaling pathway in FRO thyroid cancer cells.Korean J Intern Med. 2014 Jul;29(4):474-81. doi: 10.3904/kjim.2014.29.4.474. Epub 2014 Jun 27. Korean J Intern Med. 2014. PMID: 25045295 Free PMC article.
-
Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators.IUBMB Life. 2009 Jan;61(1):18-26. doi: 10.1002/iub.135. IUBMB Life. 2009. PMID: 18798311 Free PMC article. Review.
-
AMPK: The energy sensor at the crossroads of aging and cancer.Semin Cancer Biol. 2024 Nov;106-107:15-27. doi: 10.1016/j.semcancer.2024.08.002. Epub 2024 Aug 26. Semin Cancer Biol. 2024. PMID: 39197808 Review.
Cited by
-
Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation.Pharmaceutics. 2021 Mar 4;13(3):330. doi: 10.3390/pharmaceutics13030330. Pharmaceutics. 2021. Retraction in: Pharmaceutics. 2023 May 30;15(6):1623. doi: 10.3390/pharmaceutics15061623. PMID: 33806415 Free PMC article. Retracted.
-
Lack of vitamin D signalling shifts skeletal muscles towards oxidative metabolism.J Cachexia Sarcopenia Muscle. 2024 Feb;15(1):67-80. doi: 10.1002/jcsm.13378. Epub 2023 Dec 2. J Cachexia Sarcopenia Muscle. 2024. PMID: 38041597 Free PMC article.
-
(-)-Epicatechin-3-O-β-D-allopyranoside from Davallia formosana, Prevents Diabetes and Hyperlipidemia by Regulation of Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice.Int J Mol Sci. 2015 Oct 20;16(10):24983-5001. doi: 10.3390/ijms161024983. Int J Mol Sci. 2015. PMID: 26492243 Free PMC article.
-
Mitoenergetic Dysfunction Triggers a Rapid Compensatory Increase in Steady-State Glucose Flux.Biophys J. 2015 Oct 6;109(7):1372-86. doi: 10.1016/j.bpj.2015.08.002. Biophys J. 2015. PMID: 26445438 Free PMC article.
-
Berberine acutely activates the glucose transport activity of GLUT1.Biochimie. 2011 Jul;93(7):1187-92. doi: 10.1016/j.biochi.2011.04.013. Epub 2011 Apr 29. Biochimie. 2011. PMID: 21545824 Free PMC article.
References
-
- Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, Witters LA, Ismail-Beigi F. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys 380: 347–352, 2000. - PubMed
-
- Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology 146: 3773–3781, 2005. - PubMed
-
- Asahi Y, Hayashi H, Wang L, Ebina Y. Co-localization of the translocated GLUT4 with rearranged actin by insulin treatment in CHO cells and L6 myotubes. J Med Invest 46 :192–199, 1999. - PubMed
-
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer Lee GD, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic, and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442, 2002. - PubMed
-
- Begonja AJ, Geiger J, Rukoyatkina N, Rauchfuss S, Gambaryan S, Walter U. Thrombin stimulation of p38 MAP kinase in human platelets is mediated by ADP, and thromboxane A2 and inhibited by cGMP/cGMP-dependent protein kinase. Blood 109: 616–618, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

