Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies
- PMID: 18701681
- PMCID: PMC6670564
- DOI: 10.1523/JNEUROSCI.2218-08.2008
Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies
Abstract
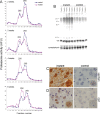
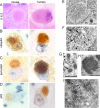
Ubiquitin-positive intraneuronal inclusions are a consistent feature of the major human neurodegenerative diseases, suggesting that dysfunction of the ubiquitin proteasome system is central to disease etiology. Research using inhibitors of the 20S proteasome to model Parkinson's disease is controversial. We report for the first time that specifically 26S proteasomal dysfunction is sufficient to trigger neurodegenerative disease. Here, we describe novel conditional genetic mouse models using the Cre/loxP system to spatially restrict inactivation of Psmc1 (Rpt2/S4) to neurons of either the substantia nigra or forebrain (e.g., cortex, hippocampus, and striatum). PSMC1 is an essential subunit of the 26S proteasome and Psmc1 conditional knock-out mice display 26S proteasome depletion in targeted neurons, in which the 20S proteasome is not affected. Impairment of specifically ubiquitin-mediated protein degradation caused intraneuronal Lewy-like inclusions and extensive neurodegeneration in the nigrostriatal pathway and forebrain regions. Ubiquitin and alpha-synuclein neuropathology was evident, similar to human Lewy bodies, but interestingly, inclusion bodies contained mitochondria. We support this observation by demonstrating mitochondria in an early form of Lewy body (pale body) from Parkinson's disease patients. The results directly confirm that 26S dysfunction in neurons is involved in the pathology of neurodegenerative disease. The model demonstrates that 26S proteasomes are necessary for normal neuronal homeostasis and that 20S proteasome activity is insufficient for neuronal survival. Finally, we are providing the first reproducible genetic platform for identifying new therapeutic targets to slow or prevent neurodegeneration.
Figures



References
-
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
-
- Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. - PubMed
-
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
