Cortisol inhibits neuroplasticity induction in human motor cortex
- PMID: 18701691
- PMCID: PMC6670557
- DOI: 10.1523/JNEUROSCI.1963-08.2008
Cortisol inhibits neuroplasticity induction in human motor cortex
Abstract
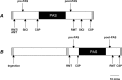
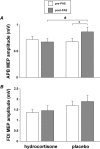
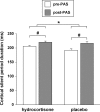
We investigated whether plasticity of human motor cortex (M1) is influenced by time of day, and whether changes in circulating levels of cortisol contribute to this effect. Neuroplasticity was induced using paired associative stimulation (PAS), involving electrical stimulation of left median nerve, paired with transcranial magnetic stimulation over the right M1 25 ms later (90 pairs at 0.05 Hz). Surface EMG was recorded from the left abductor pollicis brevis (APB) and first dorsal interosseous muscle. Cortisol levels were assessed from saliva. Time-of-day modulation of PAS effectiveness was assessed in 25 subjects who were tested twice, at 8:00 A.M. and 8:00 P.M. on separate days. In a second double-blind study, 17 subjects were tested with PAS at 8:00 P.M. on two occasions after administration of oral hydrocortisone (24 mg) or placebo. The motor-evoked potential (MEP) in resting APB increased significantly after PAS in the evening (when endogenous cortisol levels were low), but not in the morning. Oral hydrocortisone prevented facilitation of the APB MEP after PAS, and in the drug study, mean salivary cortisol levels were negatively associated with PAS effectiveness. The GABA(B)-mediated cortical silent period for APB was longer in the morning than in the evening, and was lengthened by PAS and oral hydrocortisone. We conclude that neuroplasticity in human M1 and GABA(B)-dependent intracortical inhibitory systems are influenced by time of day and modified by circulating levels of cortisol.
Figures




Similar articles
-
Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation.Exp Brain Res. 2007 Aug;181(4):615-26. doi: 10.1007/s00221-007-0960-x. Epub 2007 May 9. Exp Brain Res. 2007. PMID: 17487476
-
Induction of cortical plasticity for reciprocal muscles by paired associative stimulation.Brain Behav. 2014;4(6):822-32. doi: 10.1002/brb3.280. Epub 2014 Oct 15. Brain Behav. 2014. PMID: 25365805 Free PMC article.
-
Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals.J Physiol. 2009 Dec 15;587(Pt 24):5831-42. doi: 10.1113/jphysiol.2009.181834. J Physiol. 2009. PMID: 19858227 Free PMC article.
-
Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation.Eur J Neurosci. 2007 Jun;25(11):3461-8. doi: 10.1111/j.1460-9568.2007.05603.x. Eur J Neurosci. 2007. PMID: 17553015
-
Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation.Exp Brain Res. 2008 May;187(3):467-75. doi: 10.1007/s00221-008-1319-7. Epub 2008 Mar 5. Exp Brain Res. 2008. PMID: 18320180
Cited by
-
Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics.J Neurophysiol. 2015 May 1;113(9):3375-85. doi: 10.1152/jn.00850.2014. Epub 2015 Feb 25. J Neurophysiol. 2015. PMID: 25717162 Free PMC article.
-
Retest reliability of repetitive transcranial magnetic stimulation over the healthy human motor cortex: a systematic review and meta-analysis.Front Hum Neurosci. 2023 Sep 13;17:1237713. doi: 10.3389/fnhum.2023.1237713. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37771347 Free PMC article.
-
Two emerging concepts for elite athletes: the short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones.Sports Med. 2011 Feb 1;41(2):103-23. doi: 10.2165/11539170-000000000-00000. Sports Med. 2011. PMID: 21244104 Review.
-
Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors.Brain Sci. 2022 Apr 20;12(5):522. doi: 10.3390/brainsci12050522. Brain Sci. 2022. PMID: 35624908 Free PMC article. Review.
-
Transcranial direct current stimulation: five important issues we aren't discussing (but probably should be).Front Syst Neurosci. 2014 Jan 24;8:2. doi: 10.3389/fnsys.2014.00002. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24478640 Free PMC article. Review.
References
-
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. - PubMed
-
- Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods. 2001;112:193–202. - PubMed
-
- Castañeda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. - PubMed
-
- Charlton CS, Ridding MC, Thompson PD, Miles TS. Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J Neurol Sci. 2003;208:79–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
