Multifactorial analysis of predictors of outcome in pediatric intracranial ependymoma
- PMID: 18701711
- PMCID: PMC2666244
- DOI: 10.1215/15228517-2008-036
Multifactorial analysis of predictors of outcome in pediatric intracranial ependymoma
Abstract
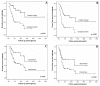
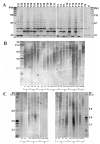
Pediatric ependymomas are enigmatic tumors, and their clinical management remains one of the more difficult in pediatric oncology. The identification of biological correlates of outcome and therapeutic targets remains a significant challenge in this disease. We therefore analyzed a panel of potential biological markers to determine optimal prognostic markers. We constructed a tissue microarray from 97 intracranial tumors from 74 patients (WHO grade II-III) and analyzed the candidate markers nucleolin, telomerase catalytic subunit (hTERT; antibody clone 44F12), survivin, Ki-67, and members of the receptor tyrosine kinase I (RTK-I) family by immunohistochemistry. Telomerase activity was determined using the in vitro-based telomere repeat amplification protocol assay, and telomere length was measured using the telomere restriction fragment assay. Primary tumors with low versus high nucleolin protein expression had a 5-year event-free survival of 74%+/-13% and 31%+/-7%, respectively. Multivariate analysis identified low nucleolin expression to be independently associated with a more favorable prognosis (hazard ratio=6.25; 95% confidence interval, 1.6-24.2; p=0.008). Ki-67 and survivin correlated with histological grade but not with outcome. Immunohistochemical detection of the RTK-I family did not correlate with grade or outcome. Telomerase activity was evident in 19 of 22 primary tumors, with telomere lengthening and/or maintenance occurring in five of seven recurrent cases. Low nucleolin expression was the single most important biological predictor of outcome in pediatric intracranial ependymoma. Furthermore, telomerase reactivation and maintenance of telomeric repeats appear necessary for childhood ependymoma progression. These findings require corroboration in a clinical trial setting.
Figures



References
-
- Goldwein JW, Leahy JM, Packer RJ, Sutton LN, Curran WJ, Rorke LB, et al. Intracranial ependymomas in children. Int J Radiat Oncol Biol Phys. 1990;19:1497–1502. - PubMed
-
- Akyuz C, Emir S, Akalan N, Soylemezoglu F, Kutluk T, Buyukpamukcu M. Intracranial ependymomas in childhood—a retrospective review of sixty-two children. Acta Oncol (Stockholm) 2000;39:97–100. - PubMed
-
- Grill J, Le Deley MC, Gambarelli D, Raquin MA, Couanet D, Pierre-Kahn A, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288–1296. - PubMed
-
- Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. NEJM. 1993;328:1725–1731. - PubMed
-
- Merchant TE, Mulhern RK, Krasin MJ, Kun LE, Williams T, Li C, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

