Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits
- PMID: 18701752
- PMCID: PMC2576194
- DOI: 10.1152/jn.90635.2008
Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits
Abstract
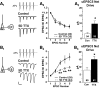
Chronic changes in neuronal activity homeostatically regulate excitatory circuitry. However, little is known about how activity regulates inhibitory circuits or specific inhibitory neuron types. Here, we examined the activity-dependent regulation of two neocortical inhibitory circuits--parvalbumin-positive (Parv+) and somatostatin-positive (Som+)--using paired recordings of synaptically coupled neurons. Action potentials were blocked for 5 days in slice culture, and unitary synaptic connections among inhibitory/excitatory neuron pairs were examined. Chronic activity blockade caused similar and distinct changes between the two inhibitory circuits. First, increases in intrinsic membrane excitability and excitatory synaptic drive in both inhibitory subtypes were consistent with the homeostatic regulation of firing rate of these neurons. On the other hand, inhibitory synapses originating from these two subtypes were differentially regulated by activity blockade. Parv+ unitary inhibitory postsynaptic current (uIPSC) strength was decreased while Som+ uIPSC strength was unchanged. Using short-duration stimulus trains, short-term plasticity for both unitary excitatory postsynaptic current (uEPSCs) and uIPSCs was unchanged in Parv+ circuitry while distinctively altered in Som+ circuitry--uEPSCs became less facilitating and uIPSCs became more depressing. In the context of recurrent inhibition, these changes would result in a frequency-dependent shift in the relative influence of each circuit. The functional changes at both types of inhibitory connections appear to be mediated by increases in presynaptic release probability and decreases in synapse number. Interestingly, these opposing changes result in decreased Parv+-mediated uIPSCs but balance out to maintain normal Som+-mediated uIPSCs. In summary, these results reveal that inhibitory circuitry is not uniformly regulated by activity levels and may provide insight into the mechanisms of both normal and pathological neocortical plasticity.
Figures







References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. - PubMed
-
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003. - PubMed
-
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420: 414–418, 2002. - PubMed
-
- Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron 42: 889–896, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

