Enhancing transduction of the liver by adeno-associated viral vectors
- PMID: 18701909
- PMCID: PMC2615795
- DOI: 10.1038/gt.2008.137
Enhancing transduction of the liver by adeno-associated viral vectors
Abstract
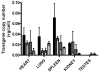
A number of distinct factors acting at different stages of the adeno-associated virus vector (AAV)-mediated gene transfer process were found to influence murine hepatocyte transduction. Foremost among these was the viral capsid protein. Self-complementary (sc) AAV pseudotyped with capsid from serotype 8 or rh.10 mediated fourfold greater hepatocyte transduction for a given vector dose when compared with vector packaged with AAV7 capsid. An almost linear relationship between vector dose and transgene expression was noted for all serotypes with vector doses as low as 1 x 10(7) vg per mouse (4 x 10(8) vg kg(-1)) mediating therapeutic levels of human FIX (hFIX) expression. Gender significantly influenced scAAV-mediated transgene expression, with twofold higher levels of expression observed in male compared with female mice. Pretreatment of mice with the proteasome inhibitor bortezomib increased scAAV-mediated hFIX expression from 4+/-0.6 to 9+/-2 microg ml(-1) in female mice, although the effect of this agent was less profound in males. Exposure of mice to adenovirus 10-20 weeks after gene transfer with AAV vectors augmented AAV transgene expression twofold by increasing the level of proviral mRNA. Hence, optimization of individual steps in the AAV gene transfer process can further enhance the potency of AAV-mediated transgene expression, thus increasing the probability of successful gene therapy.
Figures


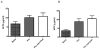





Similar articles
-
Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application.Mol Ther. 2010 Nov;18(11):1907-16. doi: 10.1038/mt.2010.170. Epub 2010 Aug 10. Mol Ther. 2010. PMID: 20700109 Free PMC article.
-
Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction.Mol Ther. 2010 Jan;18(1):135-42. doi: 10.1038/mt.2009.257. Epub 2009 Nov 10. Mol Ther. 2010. PMID: 19904235 Free PMC article.
-
Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins.Mol Ther. 2011 May;19(5):876-85. doi: 10.1038/mt.2010.274. Epub 2011 Jan 18. Mol Ther. 2011. PMID: 21245849 Free PMC article.
-
The complex and evolving story of T cell activation to AAV vector-encoded transgene products.Mol Ther. 2011 Jan;19(1):16-27. doi: 10.1038/mt.2010.250. Epub 2010 Nov 30. Mol Ther. 2011. PMID: 21119617 Free PMC article. Review.
-
The Skeletal Muscle Environment and Its Role in Immunity and Tolerance to AAV Vector-Mediated Gene Transfer.Curr Gene Ther. 2015;15(4):381-94. doi: 10.2174/1566523215666150630121750. Curr Gene Ther. 2015. PMID: 26122097 Free PMC article. Review.
Cited by
-
Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation.Mol Ther. 2009 Sep;17(9):1548-54. doi: 10.1038/mt.2009.139. Epub 2009 Jun 30. Mol Ther. 2009. PMID: 19568224 Free PMC article.
-
Promoterless gene targeting without nucleases rescues lethality of a Crigler-Najjar syndrome mouse model.EMBO Mol Med. 2017 Oct;9(10):1346-1355. doi: 10.15252/emmm.201707601. EMBO Mol Med. 2017. PMID: 28751579 Free PMC article.
-
Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application.Mol Ther. 2010 Nov;18(11):1907-16. doi: 10.1038/mt.2010.170. Epub 2010 Aug 10. Mol Ther. 2010. PMID: 20700109 Free PMC article.
-
AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage.Blood. 2012 Jan 26;119(4):957-66. doi: 10.1182/blood-2011-09-377630. Epub 2011 Dec 1. Blood. 2012. PMID: 22134170 Free PMC article.
-
Allometric-like scaling of AAV gene therapy for systemic protein delivery.Mol Ther Methods Clin Dev. 2022 Oct 21;27:368-379. doi: 10.1016/j.omtm.2022.10.011. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36381306 Free PMC article.
References
-
- Nathwani AC, Benjamin R, Nienhuis AW, Davidoff AM. Current status and prospects for gene therapy. Vox Sang. 2004 Aug;87(2):73–81. - PubMed
-
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003 Apr 15;101(8):2963–72. - PubMed
-
- Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006 Feb 12; - PubMed
-
- Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004 Jan;15(1):93–128. - PubMed
-
- Stedman H, Wilson JM, Finke R, Kleckner AL, Mendell J. Phase I clinical trial utilizing gene therapy for limb girdle muscular dystrophy: alpha-, beta-, gamma-, or delta-sarcoglycan gene delivered with intramuscular instillations of adeno-associated vectors. Hum Gene Ther. 2000 Mar 20;11(5):777–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

