Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas' disease
- PMID: 18702010
- PMCID: PMC2659458
- DOI: 10.1080/10799890802262319
Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas' disease
Abstract
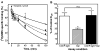
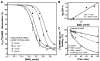
Chronic Chagasic patient immunoglobulins (CChP-IgGs) recognize an acidic amino acid cluster at the second extracellular loop (el2) of cardiac M(2)-muscarinic acetylcholine receptors (M(2)AChRs). These residues correspond to a common binding site for various allosteric agents. We characterized the nature of the M(2)AChR/CChP-IgG interaction in functional and radioligand binding experiments applying the same mainstream strategies previously used for the characterization of other allosteric agents. Dose-response curves of acetylcholine effect on heart rate were constructed with data from isolated heart experiments in the presence of CChP or normal blood donor (NBD) sera. In these experiments, CChP sera but not NBD sera increased the efficacy of agonist action by augmenting the onset of bradyarrhythmias and inducing a Hill slope of 2.5. This effect was blocked by gallamine, an M(2)AChR allosteric antagonist. Correspondingly, CChP-IgGs increased acetylcholine affinity twofold and showed negative cooperativity for [(3)H]-N-methyl scopolamine ([(3)H]-NMS) in allosterism binding assays. A peptide corresponding to the M(2)AChR-el2 blocked this effect. Furthermore, dissociation assays showed that the effect of gallamine on the [(3)H]-NMS off-rate was reverted by CChP-IgGs. Finally, concentration-effect curves for the allosteric delay of W84 on [(3)H]-NMS dissociation right shifted from an IC(50) of 33 nmol/L to 78 nmol/L, 992 nmol/L, and 1670 nmol/L in the presence of 6.7 x 10(- 8), 1.33 x 10(- 7), and 2.0 x 10(- 7) mol/L of anti-el2 affinity-purified CChP-IgGs. Taken together, these findings confirmed a competitive interplay of these ligands at the common allosteric site and revealed the novel allosteric nature of the interaction of CChP-IgGs at the M(2)AChRs as a positive cooperativity effect on acetylcholine action.
Figures
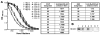

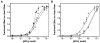

Similar articles
-
Allosteric site in M2 acetylcholine receptors: evidence for a major conformational change upon binding of an orthosteric agonist instead of an antagonist.Naunyn Schmiedebergs Arch Pharmacol. 2006 Jan;372(4):267-76. doi: 10.1007/s00210-005-0023-4. Epub 2005 Dec 16. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16362429
-
Using a radioalloster to test predictions of the cooperativity model for gallamine binding to the allosteric site of muscarinic acetylcholine M(2) receptors.Mol Pharmacol. 1999 Nov;56(5):962-5. doi: 10.1124/mol.56.5.962. Mol Pharmacol. 1999. PMID: 10531401
-
Modes of allosteric interactions with free and [3H]N-methylscopolamine-occupied muscarinic M2 receptors as deduced from buffer-dependent potency shifts.Naunyn Schmiedebergs Arch Pharmacol. 2000 Dec;362(6):512-9. doi: 10.1007/s002100000316. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 11138843
-
Allosteric interactions at muscarinic cholinoceptors.Clin Exp Pharmacol Physiol. 1998 Mar-Apr;25(3-4):185-94. doi: 10.1111/j.1440-1681.1998.t01-4-.x. Clin Exp Pharmacol Physiol. 1998. PMID: 9590567 Review.
-
G-protein coupled receptors as allosteric machines.Recept Channels. 2004;10(2):51-60. doi: 10.1080/10606820490464316. Recept Channels. 2004. PMID: 15204035 Review.
Cited by
-
Atrial standstill due to eosinophilic myocarditis and drug reaction with eosinophilia and systemic symptoms.HeartRhythm Case Rep. 2024 Dec 3;11(3):229-233. doi: 10.1016/j.hrcr.2024.11.019. eCollection 2025 Mar. HeartRhythm Case Rep. 2024. PMID: 40182941 Free PMC article. No abstract available.
-
Autoantibodies as Endogenous Modulators of GPCR Signaling.Trends Pharmacol Sci. 2021 Mar;42(3):135-150. doi: 10.1016/j.tips.2020.11.013. Epub 2020 Dec 24. Trends Pharmacol Sci. 2021. PMID: 33358695 Free PMC article. Review.
-
The Role of Autoantibodies in Arrhythmogenesis.Curr Cardiol Rep. 2020 Nov 25;23(1):3. doi: 10.1007/s11886-020-01430-x. Curr Cardiol Rep. 2020. PMID: 33241446 Free PMC article. Review.
-
Immunoglobulin G from breast cancer patients in stage I stimulates muscarinic acetylcholine receptors in MCF7 cells and induces proliferation. Participation of nitric oxide synthase-derived nitric oxide.J Clin Immunol. 2010 May;30(3):474-84. doi: 10.1007/s10875-010-9370-0. Epub 2010 Feb 16. J Clin Immunol. 2010. PMID: 20157846
-
Rational design of dualsteric GPCR ligands: quests and promise.Br J Pharmacol. 2010 Mar;159(5):997-1008. doi: 10.1111/j.1476-5381.2009.00601.x. Epub 2010 Feb 5. Br J Pharmacol. 2010. PMID: 20136835 Free PMC article. Review.
References
-
- Schmunis GA. Chagas' Disease and the Nervous System Scientific publication. Vol. 547. Washington, DC: Pan- American Health Organization; 1994. American Tripanosomiasis as a public health problem; pp. 3–31.
-
- Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: Microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11– 25. - PubMed
-
- Mason JW. Myocarditis and dilated cardiomyopathy: An inflammatory link. Cardiovasc Res. 2003;60:5–10. - PubMed
-
- Chiale PA, Ferrari I, Mahler E, Vallazza MA, Elizari MV, Rosenbaum MB, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. - PubMed
-
- Matsui S, Fu ML. Myocardial injury due to G-protein coupled receptor- autoimmunity. Jpn Heart J. 1998;39:261–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
