Novel human antibody therapeutics: the age of the Umabs
- PMID: 18702090
- PMCID: PMC2959493
- DOI: 10.1002/biot.200800110
Novel human antibody therapeutics: the age of the Umabs
Abstract
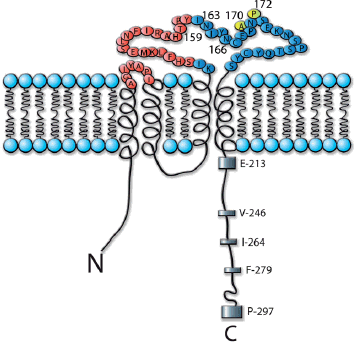
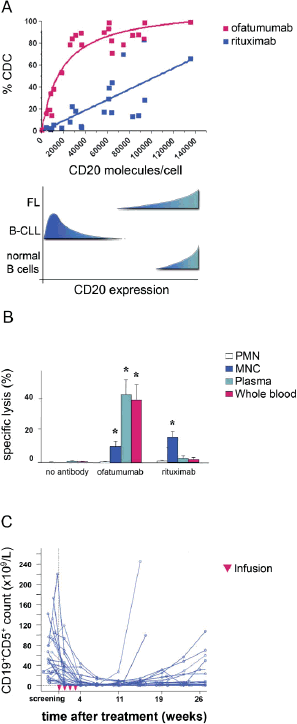
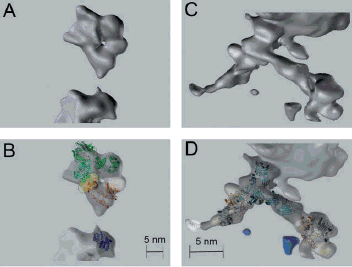
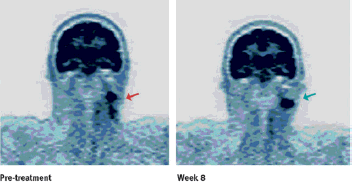
Monoclonal antibodies represent a major and increasingly important category of biotechnology products for the treatment of human diseases. The state-of-the-art of antibody technology has evolved to the point where therapeutic monoclonal antibodies, that are practically indistinguishable from antibodies induced in humans, are routinely generated. We depict how our science-based approach can be used to further improve the efficacy of antibody therapeutics, illustrated by the development of three monoclonal antibodies for various cancer indications: zanolimumab (directed against CD4), ofatumumab (directed against CD20) and zalutumumab (directed against epidermal growth factor receptor).
Figures



References
-
- Ortho Multicenter Transplant Study Group. A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N. Engl. J. Med. 1985;313:337–342. - PubMed
-
- Jones PT, Dear PH, Foote J, Neuberger MS, et al. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–525. - PubMed
-
- Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005;23:1117–1125. - PubMed
-
- Fishwild DM, O'Donnell SL, Bengoechea T, Hudson DV, et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 1996;14:845–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

