Structural investigation of a phosphorylation-catalyzed, isoaspartate-free, protein succinimide: crystallographic structure of post-succinimide His15Asp histidine-containing protein
- PMID: 18702519
- PMCID: PMC2732578
- DOI: 10.1021/bi800847a
Structural investigation of a phosphorylation-catalyzed, isoaspartate-free, protein succinimide: crystallographic structure of post-succinimide His15Asp histidine-containing protein
Abstract
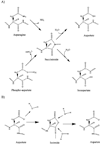
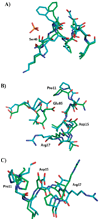
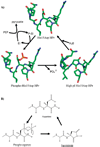
Aspartates and asparagines can spontaneously cyclize with neighboring main-chain amides to form succinimides. These succinimides hydrolyze to a mixture of isoaspartate and aspartate products. Phosphorylation of aspartates is a common mechanism of protein regulation and increases the propensity for succinimide formation. Although typically regarded as a form of protein damage, we hypothesize succinimides could represent an effective mechanism of phosphoaspartate autophosphatase activity, provided hydrolysis is limited to aspartate products. We previously reported the serendipitous creation of a protein, His15Asp histidine-containing protein (HPr), which undergoes phosphorylation-catalyzed formation of a succinimide whose hydrolysis is seemingly exclusive for aspartate formation. Here, through the high-resolution structure of postsuccinimide His15Asp HPr, we confirm the absence of isoaspartate residues and propose mechanisms for phosphorylation-catalyzed succinimide formation and its directed hydrolysis to aspartate. His15Asp HPr represents the first characterized protein example of an isoaspartate-free succinimide and lends credence to the hypothesis that intramolecular cyclization could represent a physiological mechanism of autophosphatase activity. Furthermore, this indicates that current strategies for succinimide evaluation, based on isoaspartate detection, underestimate the frequencies of these reactions. This is considerably significant for evaluation of protein stability and integrity.
Figures


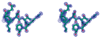

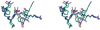
Similar articles
-
The influence of protein structure on the products emerging from succinimide hydrolysis.J Biol Chem. 2002 Aug 23;277(34):30502-7. doi: 10.1074/jbc.M205314200. Epub 2002 Jun 14. J Biol Chem. 2002. PMID: 12068021
-
The aspartyl replacement of the active site histidine in histidine-containing protein, HPr, of the Escherichia coli Phosphoenolpyruvate:Sugar phosphotransferase system can accept and donate a phosphoryl group. Spontaneous dephosphorylation of acyl-phosphate autocatalyzes an internal cyclization.J Biol Chem. 1999 Jul 30;274(31):21776-82. doi: 10.1074/jbc.274.31.21776. J Biol Chem. 1999. PMID: 10419492
-
Deamidation of asparagine residues: direct hydrolysis versus succinimide-mediated deamidation mechanisms.J Phys Chem A. 2009 Feb 12;113(6):1111-20. doi: 10.1021/jp808597v. J Phys Chem A. 2009. PMID: 19152321
-
The structure and function of HPr.Biochem Cell Biol. 1998;76(2-3):359-67. doi: 10.1139/bcb-76-2-3-359. Biochem Cell Biol. 1998. PMID: 9923705 Review.
-
Three-dimensional structures of the central regulatory proteins of the bacterial phosphotransferase system, HPr and enzyme IIAglc.J Cell Biochem. 1993 Jan;51(1):75-82. doi: 10.1002/jcb.240510114. J Cell Biochem. 1993. PMID: 8432747 Review.
Cited by
-
Characterization of the isomerization products of aspartate residues at two different sites in a monoclonal antibody.Pharm Res. 2012 Jan;29(1):187-97. doi: 10.1007/s11095-011-0534-2. Epub 2011 Aug 2. Pharm Res. 2012. PMID: 21809161
References
-
- Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int. J. Pept. Protein Res. 1987;30:808–821. - PubMed
-
- Clarke S, Stephenson RC, Lowenson JD. In: Stability of Protein Pharmaceuticals: Chemical and Physical Pathways of Protein Degradation. Ahern TJ, Manning MC, editors. New York: Plenum Press; 1992. pp. 1–29.
-
- Wright HT. CRC Crit. Rev. Biochem. Mol. Biol. 1991;27:1–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

