AMP-activated protein kinase and hypoxic pulmonary vasoconstriction
- PMID: 18703047
- PMCID: PMC3119428
- DOI: 10.1016/j.ejphar.2008.07.035
AMP-activated protein kinase and hypoxic pulmonary vasoconstriction
Abstract
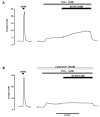
Hypoxic pulmonary vasoconstriction is a vital homeostatic mechanism that aids ventilation-perfusion matching in the lung, for which the underlying mechanism(s) remains controversial. However, our most recent investigations strongly suggest that hypoxic pulmonary vasoconstriction is precipitated, at least in part, by the inhibition of mitochondrial oxidative phosphorylation by hypoxia, an increase in the AMP/ATP ratio and consequent activation of AMP-activated protein kinase (AMPK). Unfortunately, these studies lacked the definitive proof that can only be provided by selectively blocking AMPK-dependent signalling cascades. The aim of the present study was, therefore, to determine the effects of the AMPK inhibitor compound C upon: (1) phosphorylation in response to hypoxia of a classical AMPK substrate, acetyl CoA carboxylase, in rat pulmonary arterial smooth muscle and (2) hypoxic pulmonary vasoconstriction in rat isolated intrapulmonary arteries. Acetyl CoA carboxylase phosphorylation was increased approximately 3 fold in the presence of hypoxia (pO(2) = 16-21 mm Hg, 1 h) and 5-aminoimidazole-4-carboxamide riboside (AICAR; 1 mM; 4 h) and in a manner that was significantly attenuated by the AMPK antagonist compound C (40 microM). Most importantly, pre-incubation of intrapulmonary arteries with compound C (40 microM) inhibited phase II, but not phase I, of hypoxic pulmonary vasoconstriction. Likewise, compound C (40 microM) inhibited constriction by AICAR (1 mM). The results of the present study are consistent with the activation of AMPK being a key event in the initiation of the contractile response of pulmonary arteries to acute hypoxia.
Figures



Similar articles
-
AMP-activated protein kinase phosphorylates transcription factors of the CREB family.J Appl Physiol (1985). 2008 Feb;104(2):429-38. doi: 10.1152/japplphysiol.00900.2007. Epub 2007 Dec 6. J Appl Physiol (1985). 2008. PMID: 18063805
-
Critical role of 5'-AMP-activated protein kinase in the stimulation of glucose transport in response to inhibition of oxidative phosphorylation.Am J Physiol Cell Physiol. 2007 Jan;292(1):C477-87. doi: 10.1152/ajpcell.00196.2006. Epub 2006 Aug 30. Am J Physiol Cell Physiol. 2007. PMID: 16943243
-
Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes.Eur J Biochem. 2002 Aug;269(15):3751-9. doi: 10.1046/j.1432-1033.2002.03074.x. Eur J Biochem. 2002. PMID: 12153572
-
AMP-activated protein kinase underpins hypoxic pulmonary vasoconstriction and carotid body excitation by hypoxia in mammals.Exp Physiol. 2006 Sep;91(5):821-7. doi: 10.1113/expphysiol.2006.033514. Epub 2006 Jun 1. Exp Physiol. 2006. PMID: 16740641 Review.
-
Modulation of the LKB1-AMPK Signalling Pathway Underpins Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension.Adv Exp Med Biol. 2015;860:89-99. doi: 10.1007/978-3-319-18440-1_11. Adv Exp Med Biol. 2015. PMID: 26303471 Review.
Cited by
-
AMPD1 polymorphism and response to regadenoson.Pharmacogenomics. 2015 Nov;16(16):1807-15. doi: 10.2217/pgs.15.116. Epub 2015 Nov 10. Pharmacogenomics. 2015. PMID: 26554440 Free PMC article.
-
Regulatory effect of AMP-activated protein kinase on pulmonary hypertension induced by chronic hypoxia in rats: in vivo and in vitro studies.Mol Biol Rep. 2014 Jun;41(6):4031-41. doi: 10.1007/s11033-014-3272-9. Epub 2014 Feb 25. Mol Biol Rep. 2014. PMID: 24566685
-
Impairment of hypoxic pulmonary vasoconstriction in acute respiratory distress syndrome.Eur Respir Rev. 2021 Sep 15;30(161):210059. doi: 10.1183/16000617.0059-2021. Print 2021 Sep 30. Eur Respir Rev. 2021. PMID: 34526314 Free PMC article. Review.
-
Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway.Nat Commun. 2018 Sep 21;9(1):3850. doi: 10.1038/s41467-018-06376-x. Nat Commun. 2018. PMID: 30242159 Free PMC article.
-
AMPK and the Challenge of Treating Hypoxic Pulmonary Hypertension.Int J Mol Sci. 2022 Jun 1;23(11):6205. doi: 10.3390/ijms23116205. Int J Mol Sci. 2022. PMID: 35682884 Free PMC article. Review.
References
-
- Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz. 1986;11:127–141. - PubMed
-
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995a;229:558–565. - PubMed
-
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995b;229:558–565. - PubMed
-
- Dipp M, Evans AM. Cyclic ADP-Ribose Is the Primary Trigger for Hypoxic Pulmonary Vasoconstriction in the Rat Lung In Situ. Circ. Res. 2001;89:77–83. - PubMed
-
- Dipp M, Nye PCG, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am. J. Physiol. 2001;281:L318–L325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

